Figure 2: eCIRP-induced wild-type, but not PAD4−/− neutrophils inhibit efferocytosis.
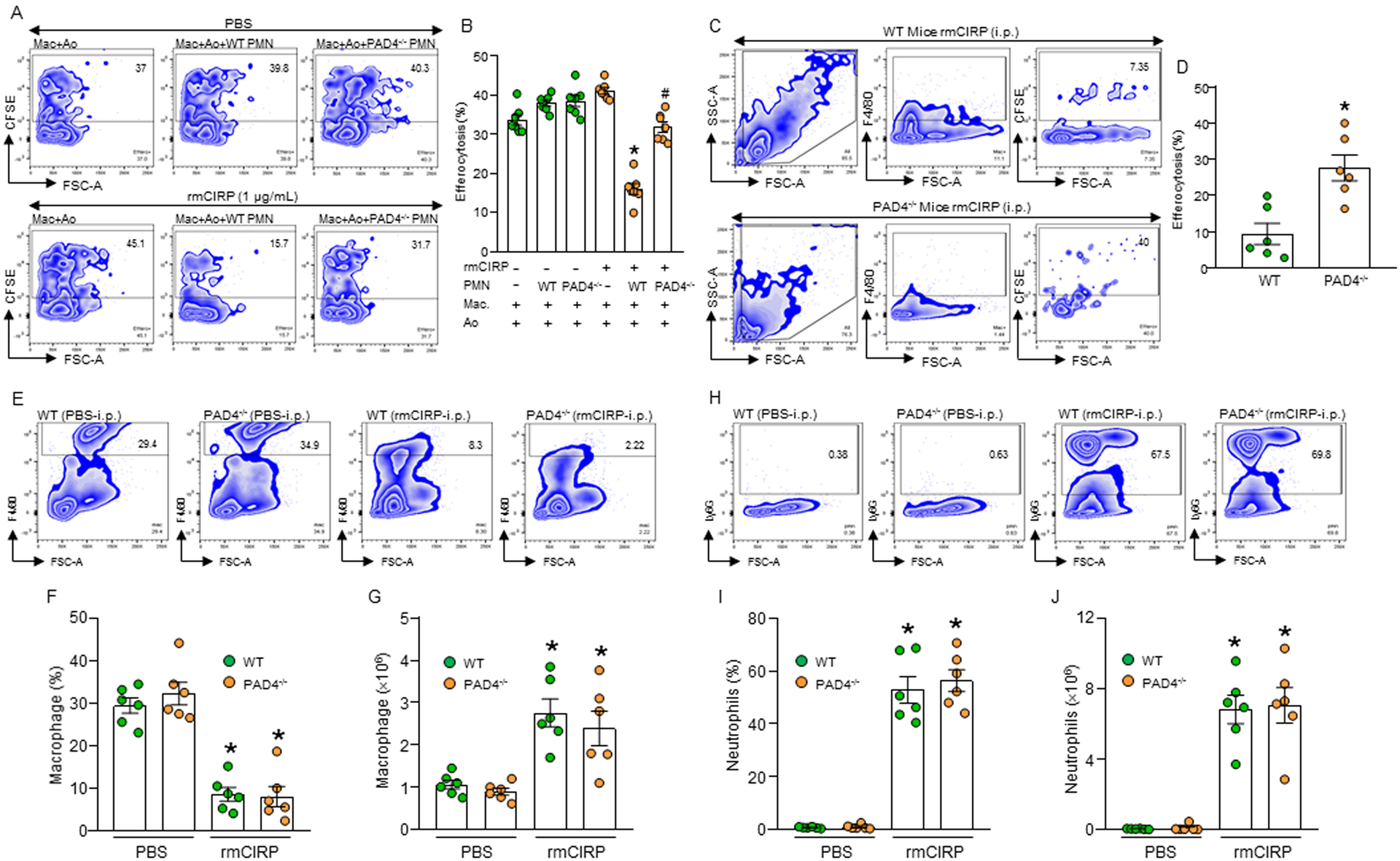
(A, B) BMDN (1× 106) isolated from WT or PAD4−/− mice were stimulated with PBS or rmCIRP (1 μg/mL) for 4 h to allow them to form NETs. Peritoneal macrophages (5 × 105) and CFSE-labeled apoptotic cells (Ao) (1.5 × 106) were separately added to PBS- or rmCIRP-treated WT or PAD4−/− PMN. Cells were continued to culture for 1 h. Cells were then stained with PE-F4/80 Abs and assessed efferocytosis by flow cytometry. Data were obtained from 3 independent experiments and expressed as means ± SE (n=7 samples/group). The groups were compared by one-way ANOVA and SNK method. *p<0.05 vs. rmCIRP(−) PMN(−); #p<0.05 vs. rmCIRP(+) WT PMN(+). (C, D) WT and PAD4−/− mice were injected with rmCIRP (5 mg/kg; i.p.). After 4 h of injection with rmCIRP, a total of 1 × 107 CFSE-labeled apoptotic cells were injected i.p. into the mice. After 1 h, peritoneal washout cells were collected, stained with PE-F4/80 Ab, and assessed efferocytosis by flow cytometry. Data were obtained from 3 independent experiments and expressed as means ± SE (n=6 mice/group). The groups were compared by one-way ANOVA and SNK method. *p<0.05 vs. WT mice. (E-J) WT and PAD4−/− mice were injected with rmCIRP (5 mg/kg; i.p.). After 20 h of injecting the mice with rmCIRP, peritoneal washout cells were harvested and stained with macrophage marker PE-F4/80 and neutrophil marker APC-Ly6G Abs. The contents of (E-G) macrophages and (H-J) neutrophils in the peritoneal cavity were assessed my flow cytometry. Data were obtained from 3 independent experiments and expressed as means ± SE (n=6 mice/group). The groups were compared by one-way ANOVA and SNK method. *p<0.05 vs. PBS-treated WT/PAD4−/− mice. PAD4, peptidylarginine deiminase 4; PMN, polymorphonuclear leukocytes; Ao, apoptotic cells.
