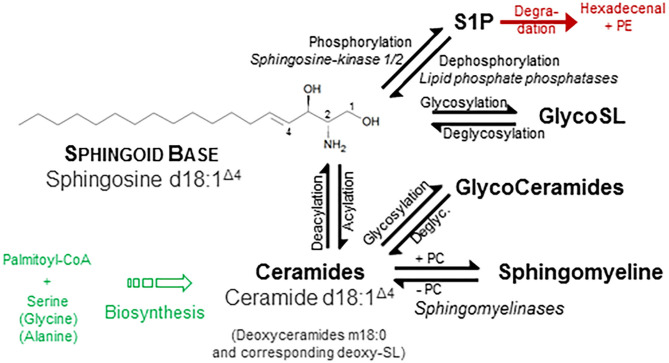
Figure 1.
Structural and physicochemical diversity of sphingolipids. Sphingolipids (SP) share a common hydrophobic Sphingoid Base structure, which most abundant species is Sphingosine (d18:1 Δ4) (1). Diversity of SP originates from either discrete or common substitution at hydroxyl moiety (Pos. 1) as well as at the amino moiety (Pos. 2) and—more categorical—variation of the backbone with respect to number and position of hydroxyl groups and unsaturated bonds (not shown). Discrete phosphorylation of the hydroxyl group at position 1 results in the simple sphingoid base derivatives Sphingosine-1-Phosphate (S1P), acting at a family of G-protein-coupled S1P-receptors. Acylation of the amino moiety at position two establish the N-acylated sphingoid bases, termed Ceramides (Cer), where the fatty acid also varies in length (16–26 carbon atoms), hydroxylation, and saturation rate in a broad range. Esterification of these ceramides with phosphorylcholine (PC) results in the phosphono series of SP, esp. Sphingomyelines. Additionally, glycosidic bonding of the hydroxyl group with mono- up to tetra-saccharides with different compositions initiates the most complex group of Glycosphingolipids, which are considered as derivatives of ceramide. For instance, a β-glycosidical linkage of D-glucose to the 1-hydroxyl moiety of ceramide results in formation of glucosylceramide (GlcCer). Ceramides, glycosphingolipids, and SM do not display a net charge and are therefore neutral sphingolipids, whereas linkage of glyco-sphingolipids with sialic acid or sulfate is negatively charged. Other sphingoid bases differ in number of unsaturated bonds (Sphinganine d18:0, syn. dihydrosphingosine; Sphingadiene d18:2 Δ4, 14) as well as number and position of hydroxyl groups: Phytosphingosine t18:0 and Deoxysphingosine m18:0. Use of alanine instead of serine as a building block in the first condensation step produces deoxysphingosine derivatives (m18:1). A plethora of additional variations are found in other organisms (2). Along chemical structures of individual lipids and their derivatives, categorization into distinct classes and subclasses, nomenclature, and cataloging of (sphingo-)lipids and of their properties were performed by the LIPID MAPS Consortium (3), most recently revised by leading authorities in the field (4). Similar to sphingosine, also Cer is phosphorylated to the bioactive lipid mediator Cer-1-phosphate (not shown) (5). Also, major SP classes are metabolically interconvertible by enzyme-mediated pathways (6, 7), some names of which are given in italic style.