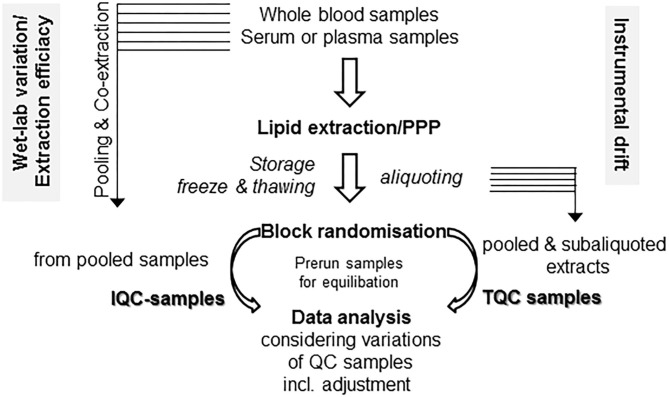
Figure 2.
Monitoring technical and process related variations. With two distinct sets of quality control (QC) samples, there is a clear differentiation between drifts caused due to “wet-lab” non-conformance during sample preparation (in-process QC, IQC) as well as by instrumental changes (technical QC, TQC). For these issues, two separate sets of QC samples might be prepared. For IQC preparation, you gather (a portion of) the raw starting material (i.e., plasma/serum) prior to extraction and co-extract these aliquots in similar conditions with the samples in your preanalytical preparation procedure (PPP) workflow. The second set is gathered from the already reconstituted solutions following PPP from either a single batch or a subcohort, then pooled and aliquoted into separate vials (TQC) and should display a representative pool of your analysis. Run these samples at the beginning, at the end, and in the long run in an intermittent manner. Use of biostatistical algorithms, e.g., principal component analysis, will demonstrate deviations from conformity and help to control adjustment procedures (123). For equilibration of best performance separation condition, use pre-run samples with similar matrix components, which will not be included in data analysis.