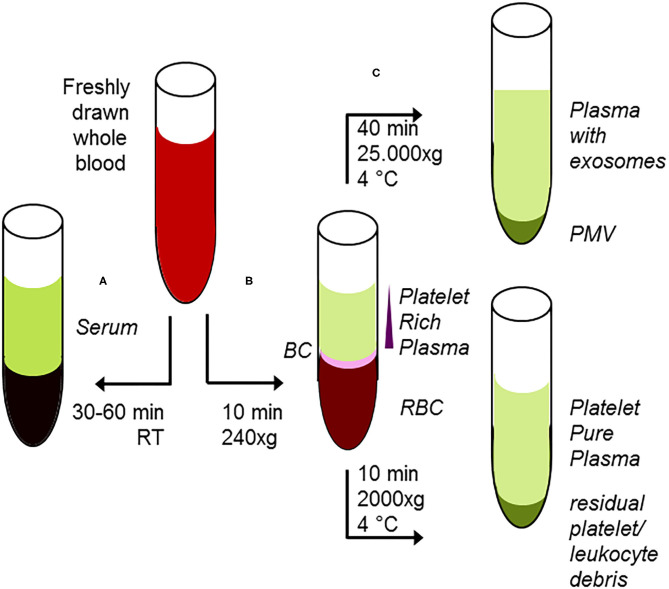
Figure 3.
Classical protocol for preanalytical procedures of starting material. (A) After adding of clotting enhancers to freshly drawn blood and completion of clotting procedure 30–60 min, room temperature), serum samples are harvested following centrifugation. (B) Freshly drawn, anti-coagulated whole blood is briefly centrifuged at low centrifugal forces (softspin, 240 × g, 10 min), resulting in separation of three layers: red blood cells (RBC) at the bottom, a layer termed “buffy coat” (BC) of typically whitish color containing the major proportion of leucocytes, and some platelets in an intermediate layer and acellular, crude platelet-rich plasma fraction (PRP) with varying platelet contents. For further separation, the crude PRP layer is transferred to a fresh tube. After hard-spin centrifugation, most of the supernatant is harvested as the platelet-pure plasma fraction. The final PRP concentrate at the bottom consists of an undetermined fraction of BC (containing a large number of platelets) suspended in some fibrin-rich plasma. The transfer step is often performed with a syringe or pipette, with only visual inspection. Because the manual PPP process is not clearly defined, this protocol might randomly lead to impure plasma fractions. (C) From the PRP fraction, platelet-derived microvesicles (PMV) are isolated by centrifugation (25.000 × g/40 min), and finally, plasma-borne exosomes can be obtained from the remaining supernatant. Using the buffy coat fraction for isolation of leukocytes, nucleated cells should be further purified by a second centrifugation step.