Abstract
Background:
Because atherosclerosis begins in childhood, universal lipid screening is recommended with special attention to conditions predisposing to early atherosclerosis. Data about real-world penetration of these guidelines is not available.
Methods:
Retrospective cohort study using MarketScan® commercial and Medicaid insurance claims databases, a geographically representative sample of U.S. children. Subjects who passed through the 9- to 11-year window and had continuous insurance coverage between 1/1/2013–12/31/2016 were studied. Multivariable models were calculated, evaluating the association between other patient factors and the likelihood of screening. The primary hypothesis was that screening rates would be low, but that high-risk conditions would be associated with a higher likelihood of screening.
Results:
In total, 572,522 children (51% male, 33% black, 11% Hispanic, 51% Medicaid) were studied. The prevalence of high-risk conditions was 2.2%. In unadjusted and adjusted analyses, these subjects were more likely to be screened than standard-risk subjects (47% vs. 20%, OR: 3.7, 95% CI 3.5–3.8, P<0.001). Within this group, the diagnosis-specific likelihood of screening varied (26–69%). Endocrinopathies (OR 5.4, 95% CI 5.2–5.7), solid organ transplants (OR 5.0, 95% CI 3.8–6.6), and metabolic disease (OR 3.9, 95% CI 3.1–5.0, all P<0.001) were associated with the highest likelihood of undergoing screening.
Conclusions:
Despite national recommendations, lipid screening was performed in a minority of children. Though subjects with high-risk conditions had a higher likelihood of screening, rates remained low. This study highlights the need for research and advocacy regarding obstacles to lipid screening of children in the U.S.
Introduction
Cardiovascular disease (CVD) is the leading cause of death among adults in the United States, and dyslipidemia beginning in childhood is an important risk factor in its development.1–4 Lifestyle and pharmacologic interventions aim to improve serum lipids in the hopes of slowing and/or preventing atherosclerosis from progressing to clinical CVD.5 Screening children for dyslipidemia provides an opportunity for earlier interventions, which may be particularly important for children at increased risk of early CVD.6 One critical goal of lipid screening in pediatrics is to detect children with the genetic condition heterozygous familial hypercholesterolemia, a common condition in 1 in 250 people.7 Historically, measuring serum lipid levels was recommended in “at-risk” children, such as those with high body mass index, diabetes mellitus, and/or a family history of early CVD.8 However, there is evidence that this strategy may be ineffective, missing >30% of children with clinically significant dyslipidemia.8,9 In 2011, the National Heart, Lung, and Blood Institute (NHLBI) released practice guidelines endorsed by the American Academy of Pediatrics (AAP) recommending that all children should be screened with a fasting or non-fasting lipid panel once between ages 9 and 11 and again between ages 17 and 21.8,10 This built upon 2006 screening recommendations from the AAP and American Heart Association (AHA) which advised early screening for “high-risk” patients, those with chronic medical conditions in which the likelihood of developing dyslipidemia is higher and/or the risk of developing early CVD in the presence of dyslipidemia is increased.11
There is limited data about how effective these recommendations were in achieving successful screening in children. Reported screening rates range widely between 3% and 46% in standard-risk patients. These studies were limited by small sample size from single centers or discrete geographic regions,12–15 practitioner self-reporting,16,17 or omission of high-risk populations,18,19 which may explain the broad range of estimates. To overcome these obstacles and achieve a real-world estimate of screening, we leveraged a geographically representative insurance claims database to perform a retrospective cohort study. We hypothesized that subjects with medical conditions that predisposed them to early atherosclerosis were more likely to undergo lipid screening than those with standard risk. We also sought to determine whether measurable confounders (e.g., race, insurance type, region, specific diagnoses within high-risk categories) were associated with a lower likelihood of screening.
Methods
Data Source:
We conducted a retrospective cohort study to assess for adherence to lipid screening guidelines using the MarketScan® Commercial and Medicaid Claims and Encounters Databases (MarketScan) (Truven Health Analytics, Ann Arbor, MI). The MarketScan database contains longitudinal, de-identified healthcare reimbursement data from U.S. commercial and public payers, including data spanning the entire continuum of healthcare services (inpatient, outpatient, emergency department, and pharmacy encounters). It provides one of the largest sources of patient-level, geographically representative data for children ages 0 to 18.20–25 Claims databases such as Marketscan® do not contain granular detail of registries or direct medical record review, but expediently provide a large representative sample. An advantage of the Marketscan® Database is that it combines commercial insurance with Medicaid providing a population that spans the spectrum of income. Recipients of insurance through the Children’s Health Insurance Program (with family incomes between recipients of commercial and Medicaid insurance) are not included. Receipt of Supplemental Security Income (SSI) entitles most children to Medicaid coverage; the cohort of patients with SSI but not Medicaid was not specifically assessed. A second advantage of the Marketscan® database is that data collected from multiple public and private health plans are consolidated and standardized by IBM/Watson accommodating different data structures and formats to create a comparable data set. No imputation is performed. Our Institutional Review Board determined that the study was exempt from review. This research was supported, in part, by the Cardiac Center Clinical Research Core. It was funded, in part, by an intramural grant from The Children’s Hospital of Philadelphia Cardiac Center. Dr. O’Byrne (K23 HL130420–01) and Dr. Berger (T32 HL1007915) receive support from the National Institutes of Health/National Heart Lung and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Study Population
The study period was January 1, 2013 to December 31, 2016. The two years elapsed between guideline publication and the start of the study period was, on face, a reasonable amount of time for guideline dissemination. The available data do not include other time periods for measurement of secular trends. Subjects were included if they passed through ages 9 to 11 during the study period (Supplemental Figure 1) and had continuous insurance coverage during those years. This approach prioritizes internal sample validity (avoiding missing data) over expanding the sample size while accepting the potential for introducing bias. Children with discontinuous insurance, previously shown to have a less favorable lipid profile,26 are less likely to be screened than those with continuous insurance, so measured estimates are high-bound estimates. There were no additional exclusion criteria.
Study data:
Data were directly extracted from the MarketScan database and included sex, race, ethnicity, census region, insurance type, and presence of a high-risk condition. Identification of exposures, outcomes, and covariates was made using billing codes. Subjects with conditions that predisposed them to high risk of early atherosclerosis based on 2006 AHA/AAP guidelines [renal disease, solid organ transplantation, select cardiac diseases, chronic inflammatory disease, chronic infection, exposure to chemotherapy/childhood cancer, endocrinopathies including diabetes and thyroid disease, and primary metabolic disease (inborn errors of metabolism)] were identified using International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) codes (Supplemental Table 1).11 ICD codes were based on prior validated lists for chronic pediatric disease, when appropriate.27 Obesity was not included, as it is has previously been demonstrated to not be coded well in billing data.28 Notable risk factors including family or smoking history as well as clinical measurements including blood pressure and lipid testing were not available in this database. We omitted definitions from the 2019 update since they did not apply to our study population.29 Presence of any of these codes at any encounter was considered sufficient for inclusion in the high-risk category. For subjects identified as having more than one high-risk condition, each class was recorded to allow for separate analysis. We defined standard risk by absence of a high-risk diagnosis code. The contents of commercial and Medicaid databases differed in the following ways: race/ethnicity data were only included in the Medicaid database and census region was available only in the commercial insurance dataset.
We hoped to identify the proportion of subjects who had undergone lipid testing. To this end, we created a composite outcome composed of three components: 1) record of lipid laboratory testing, 2) a filled prescription for a lipid-lowering agent, and/or 3) an ICD-9 or −10 codes for dyslipidemia. Use of lipid-lowering agents or subjects with a diagnosis of dyslipidemia were included as “screened” populations because these implied previous screening. This approach does not distinguish between “initial” versus “subsequent” screens, nor does it necessarily identify the age of first lipid screen given the guideline-derived three year window. We identified screening tests using Current Procedural Terminology codes for lipid panels and/or any of the following: cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein. Lipid-lowering agents were identified using National Drug Codes (NDC) for 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), niacin, bile acid sequestrants, fibrates, and cholesterol absorption inhibitors (ezetimibe). Newer therapeutics including proprotein convertase subtilisin/kexin type 9 or diacylglycerol acyltransferase 1 inhibitors are not currently approved for use in pediatrics, with exceptions made for those with rare genetic cholesterol disorders. Some of the above medications have potential alternative uses besides lipid-lowering (e.g. cholestyramine as an anti-diarrheal). NDC codes are updated frequently, so to ensure an accurate set of codes, we previously cross-checked the tables in Marketscan against national databases and found that it is complete.30 The vast majority of standard- and high-risk patients (89% and 74%, respectively) were included in the outcome due to lipid testing, followed by a combination of screening and lipid-related diagnosis (9% and 20%) and lipid-related diagnosis alone (2% and 3%) (Supplemental Table 2). Only small percentages had a lipid-modifying prescription or other combinations of the three components.
Data Analysis
Standard descriptive statistics were calculated. The baseline characteristics of standard- and high-risk subjects were compared using Chi squared tests. Our primary aim was to determine the proportion of children undergoing lipid screening in the timeframe recommended by NHLBI/AAP guidelines (9 to 11 years). We also evaluated whether subjects with high-risk diagnoses were more likely to undergo screening. Lastly, we sought to explain differences in screening based on associated socioeconomic variables. Observed proportions were reported including 95% confidence intervals.
Secondary analyses included determining whether 1) specific high-risk conditions and 2) patient race/ethnicity, insurance type, and census region were associated with differences in screening. Multivariable logistic regression models adjusting for measurable confounders were calculated including sex, insurance type, and presence of each of the high-risk categories. Subsequent analyses added race/ethnicity or census region independently to these models. Because of the differences in commercial and Medicaid databases, a number of approaches were taken including 1) restricting analysis for race to Medicaid and region to commercial insurance and 2) coding race for commercial insurance subjects as a “commercial insurance” and region as “Medicaid” for those subjects and removing insurance type from the latter analysis.
There were no missing data for sex, payer, or age. For region and race/ethnicity, the amount of missing data was relatively small (<10%), so we created an “other/unknown” category for analysis. All statistical analyses were performed using SAS v9.4 (SAS Corporation, Raleigh NC). The threshold for statistical significance was set at P<0.05.
Results
Study Population:
A total of 572,522 children met inclusion criteria. The cohort was 51% male with 51% receiving Medicaid (Table 1). Among recipients of Medicaid, 48% were white, 33% black, and 11% Hispanic.
Table 1.
Demographic variables for standard- versus high-risk cohorts.
| Standard risk N=559,932 | High risk N=12,590 | P | |
|---|---|---|---|
| Male sex | 51% (286,125) | 52% (6,462) | 0.62 |
| Insurance type | <0.001 | ||
| Commercial | 49% (276,149) | 40% (5,089) | |
| Medicaid | 51% (283,783) | 60% (7,501) | |
| Census region (commercial insurance only) | <0.001 | ||
| Northeast | 21% (58,984) | 21% (1,062) | |
| Midwest | 19% (53,547) | 22% (1,125) | |
| South | 43% (117,735) | 40% (2,014) | |
| West | 16% (45,234) | 17% (879) | |
| Unknown | 0.2% (649) | 0.2% (9) | |
| Race/Ethnicity (Medicaid only) | <0.001 | ||
| White | 48% (136,063) | 50% (3,718) | |
| Black | 33% (93,408) | 30% (2,211) | |
| Hispanic | 11% (30,560) | 10% (744) | |
| Unknown/Other | 8% (23,752) | 11% (828) |
Of the overall population, 2.25% had conditions placing them at increased risk of early atherosclerosis (high-risk group). Comparing standard- and high-risk groups, a lower proportion of standard-risk subjects received Medicaid than high-risk subjects (51% versus 60%, P<0.001). Among commercial insurance subjects, there were statistically significant but small differences in the distribution of subjects in census regions, specifically a higher proportion of high-risk subjects in the Northeast (22% vs. 19%, P<0.001) and smaller proportion in the South (40% vs. 43%, P<0.001). For Medicaid subjects, standard-risk subjects were more likely to be black than high-risk subjects (33% vs. 30%, P<0.001).
The most prevalent high-risk disease categories in this cohort included endocrinopathies (62%) and high-risk cardiac disease (17%) (Supplemental Table 3). Solid organ transplant status (2.2%), chronic infection (2.1%), and metabolic diseases (2.3%) were the least prevalent. The distribution of baseline characteristics was generally similar across categories. The presence of cardiac disease (63%, P<0.001), organ transplantation (58%, P<0.001), and chemotherapy (58%, P<0.001) was positively associated with male sex, while chronic inflammatory conditions (e.g., autoimmune) were positively associated with female sex (64%, P<0.001). Subjects with cardiac and metabolic disease were more likely to have commercial insurance (52% and 58%, respectively versus 49%, P<0.005) whereas subjects with renal disease, organ transplant, and endocrinopathies were more likely to have Medicaid coverage (61%, 66%, and 66%, respectively vs 51%, P<0.001 for all). There were no discernable patterns among region for commercial insurance patients or race/ethnicity for Medicaid patients across categories of chronic disease.
Screening rates:
Lipid screening was performed in 19.8% (95% CI 19.7–19.9%) of standard-risk subjects (Figure 1). This was significantly lower than in high-risk patients, of whom 47% (95% CI 46.1–47.9%, P<0.001) were screened. While the proportion of patients screened differed amongst specific high-risk conditions with a range of 26.8% to 68.7% (Figure 1), high-risk conditions were, overall, associated with higher odds of screening (OR 3.7, 95% CI 3.5–3.8, P<0.001) (Figure 2). After adjusting for measurable confounders, these differences remained. Subjects with endocrinopathies (OR 5.5, 95% CI 5.2–5.7, P<0.001), solid organ transplants (OR 5.0, 95% CI 3.8–6.6, P<0.001), and metabolic disease (OR 3.9, 95% CI 3.1–5.0, P<0.001) were associated with greater odds of screening relative to standard-risk patients (Figure 2). Odds of screening for children with cardiac disease (OR 1.3, 95% CI 1.2–1.4), chronic inflammation (OR 1.3, 95% CI 1.1–1.5), and post-chemotherapy (OR 1.4, 95% CI 1.2–1.7, all P<0.01) were all higher than standard-risk patients, but the lowest of the high-risk groups. In secondary analyses, no significant association was seen between census region, race, ethnicity, or insurance type and likelihood of being screened (data not shown).
Figure 1:
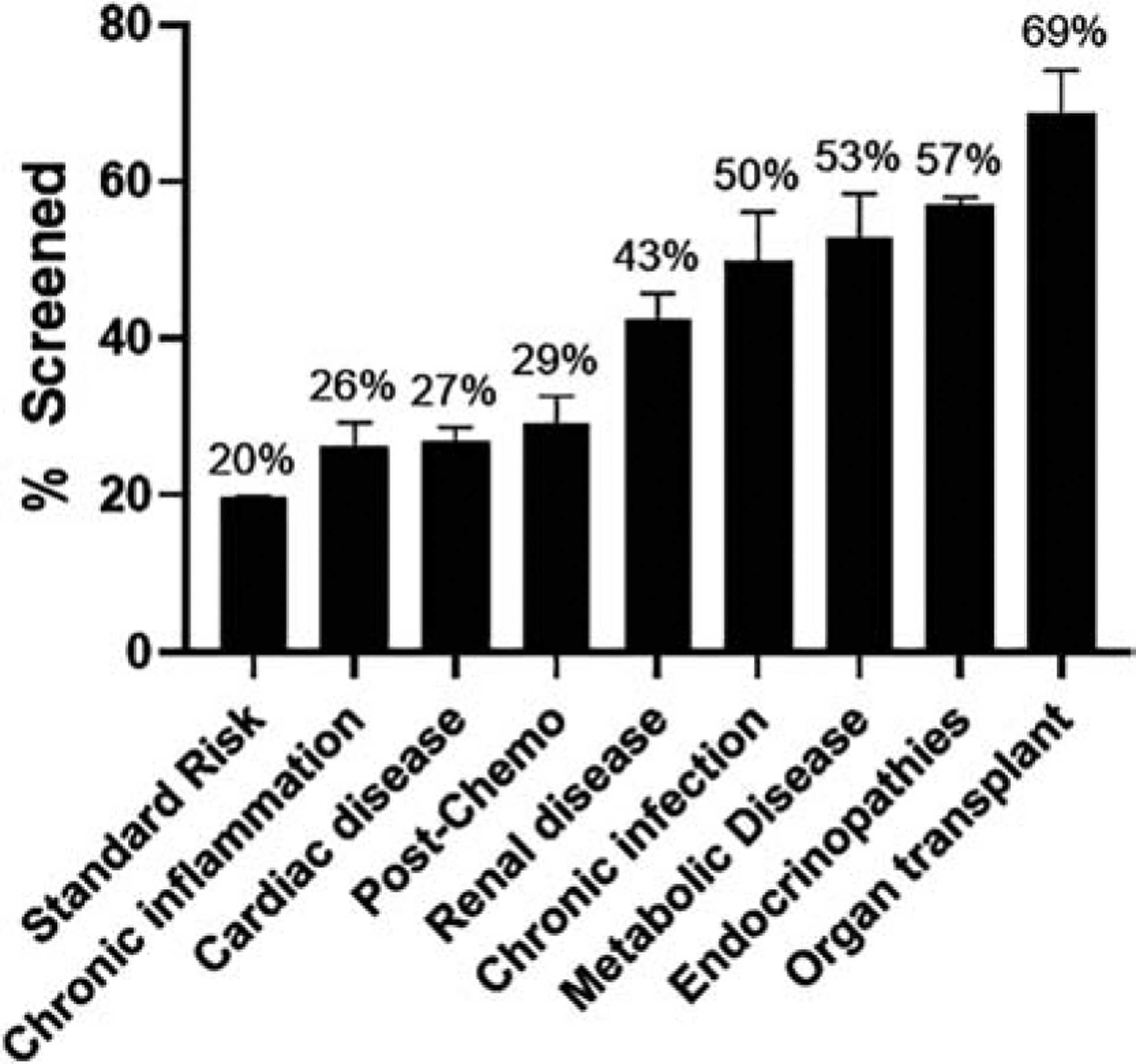
Rate of universal lipid screening is highly variable by high-risk disease cohort. Error bar represents standard deviation. Statistical significance seen in all high-risk conditions compared to standard risk (not shown graphically).
Figure 2:
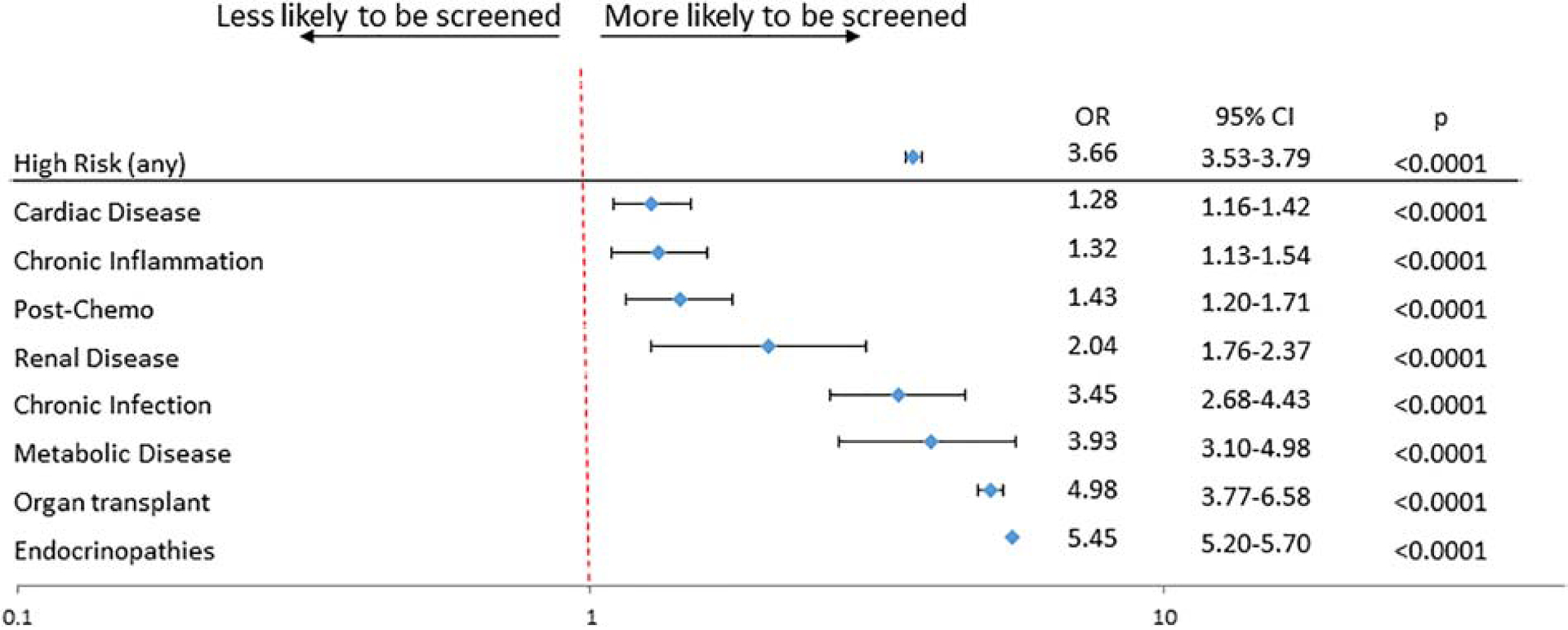
Likelihood of primary outcome varies in multivariate analysis by high-risk disease cohort. Primary outcome was composite of lipid screening, dyslipidemia diagnosis, and/or lipid-modifying therapeutic. Data compared to standard risk group and adjusted for sex, race/ethnicity, region, and payer.
Further analysis was undertaken within high-risk disease categories to better understand which patients received screening (Supplemental Table 4 and Figure 3). Among patients with chronic kidney disease (CKD), patients with severe disease (CKD stages 3–5, end-stage renal disease, or kidney transplant) had a trend toward higher occurrence of screening compared to patients with CKD stages 1–2, but the difference was not significant (P=0.78). At the same time, there were no significant differences in the proportion of screening between recipients of different solid organ transplants. Patients with diabetes were more likely to be screened (OR 7.5, 95% CI 6.9–8.2, P<0.001) than patients with other endocrine diagnoses (e.g., calcium homeostasis, polycystic ovarian syndrome). Interestingly, a group of more than 5,100 patients carried the ICD diagnosis of “metabolic syndrome.” While this entity is not commonly used as a pediatric diagnosis, those patients had an increased likelihood of receiving lipid screening (OR 4.5, 95% CI 4.3–4.8, P<0.001).
Figure 3:
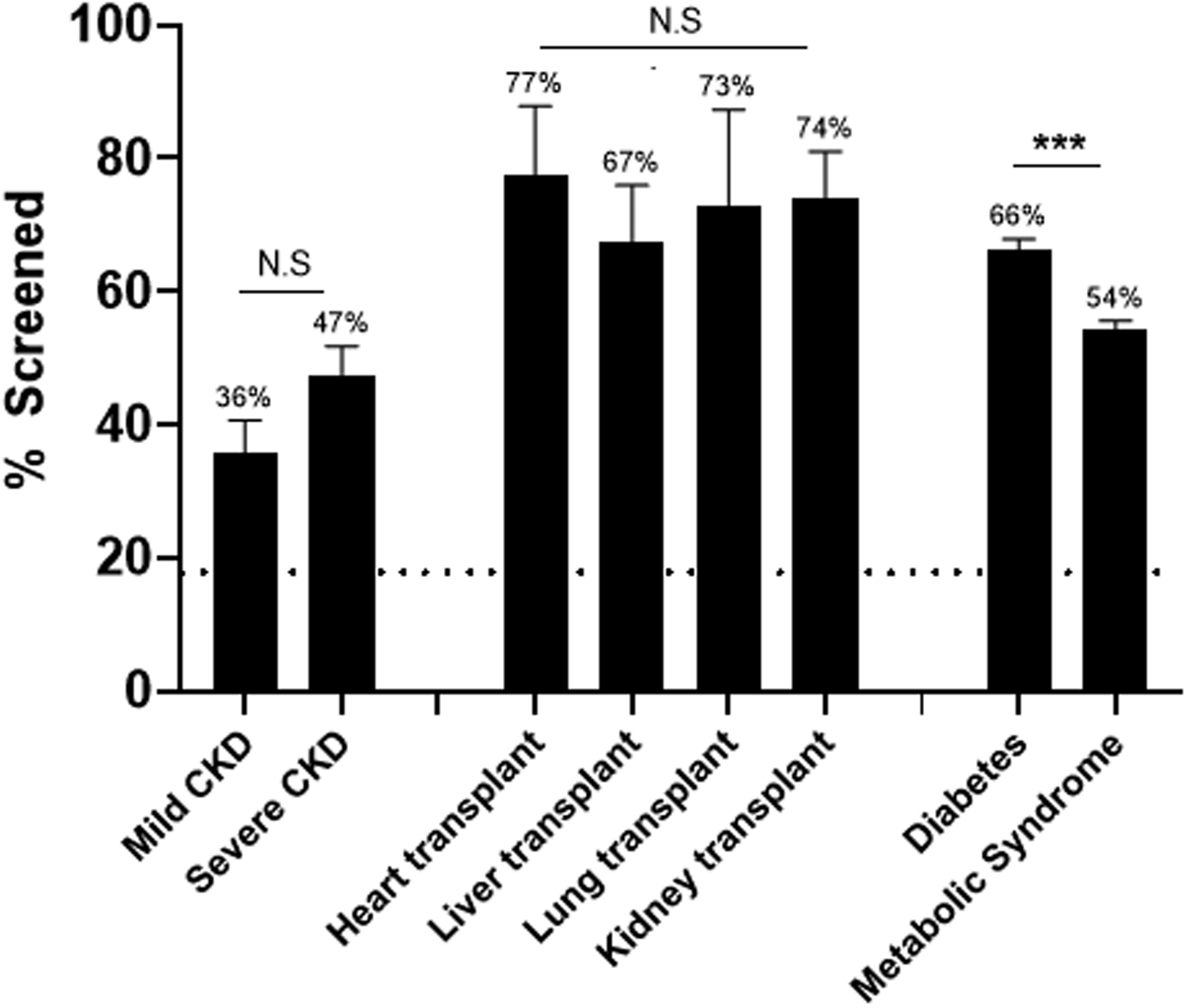
Rate of universal lipid screening among subsets of high-risk cohorts. Dashed horizontal line represents standard-risk screening percentage. Error bar represents standard deviation. ***, P<0.001; NS, not significant.
Discussion
The rationale for screening children is that early identification of at-risk populations allows for earlier, effective intervention. This principle has been shown from decades of caring for patients with heterozygous familial hypercholesterolemia.7 Because of these patients, there is increasing evidence of the safety and efficacy, and yet underutilization, of lipid lowering therapies in children.31,32 This study demonstrates that current recommendations for universal lipid screening in the United States are reaching a fraction of standard-risk children. Our reported rates are similar to what has been published in some smaller or regional studies focusing on the post-guideline time frame (16–27%)12,15 but significantly higher than other studies (3.5–11%) especially those looking prior to 2011. Though the published guidelines may not have achieved the desired universal screening, these findings suggest that they did have a measurable impact. High-risk children are more likely to undergo lipid screening, but still at levels (47%) below the goal of universal screening. These patients should also receive early (e.g., starting after age 2) and/or more frequent (e.g. at time of recognition of high-risk factor or diagnosis of high-risk disease) screening but the current study did not evaluate this and is independent of the recommendation that they should also be screened between ages 9 and 11.
There are many possible explanations for the low proportion of screening. Published data about lipid screening demonstrate that 74% of primary care clinicians believe in its efficacy, but that only a minority (36%) report adherence to recommended universal screening.16,17 It is worth noting that the publication of these guidelines were met with controversy, and there is ongoing discussion about what the guidelines should be.33–38 In these studies, a lack of familiarity with published guidelines; discomfort treating hyperlipidemia, primarily related to initiation of statin therapy; and/or lack of accessibility to lipid specialists have been identified as reasons that pediatricians fail to screen children.16,39 Prior studies have suggested patient/family follow-through (independent of cost) is also an issue.40 The economic impact of screening is also an important question beyond the scope of this study.
The strengths of this study include a large and geographical representative patient population. An important distinction from prior studies is that the current study measures the real world rates at which children are screened. An observational, insurance claims dataset provides information about what services are received by individual patients but does not provide access to provider attitudes. It also does not differentiate between providers failing to prescribe screening and families not obtaining labs. Additionally, administrative data are expedient but do not contain all clinically relevant data. Measures of body composition (specifically overweight and obesity) have specifically been shown to be unreliably coded in administrative data.28,41 Pediatric obesity is a well-established independent risk factor for dyslipidemia and CVD in children1,2,6 and there is clear evidence that pediatric obesity is associated with increased risk of adult obesity and worse adult health outcomes.42–45 Obesity is now epidemic with over one-third of children overweight or obese, and on its own should prompt screening and intervention per the NHLBI guidelines.10,46 The overlap of obesity with other high-risk diagnoses and its impact on prompting lipid screening was unable to be assessed in this study, and warrants future investigation. Differences in data reporting between different payers are addressed by the vendor without imputation. There is no indication that these differences introduce systematic differences (i.e. bias) in the data for the analyses performed. However, we conceded that this contention is not evaluable and is a potential limitation. Other limitations include a risk of unmeasured confounding or potential for misclassification of high- and standard-risk subjects, though the prevalence of high-risk conditions is comparable to published data.47,48 Because the definition of screening included subjects diagnosed with dyslipidemia, the cited rates of screening are high-bound estimates.
While accepting these limitations, we did attempt to identify any clinical and social factors associated with screening rates. In terms of clinical factors, several diagnoses were associated with increased screening rates (e.g., solid organ transplant). The current analysis cannot evaluate why this is true, but it may be worthwhile to determine how to improve guideline adherence in both high- and standard-risk populations. It is noteworthy that in some conditions (e.g., renal insufficiency), increasing severity of disease was associated with greater odds of screening, suggesting that providers are screening with an eye toward perceived risk. Specialists caring for high-risk patient populations are important stakeholders in the effort to improve screening rates. For instance, screening in congenital heart disease was low, which is consistent with reports showing that pediatric cardiologists have not commonly embraced a role in screening and counseling on risk factors for future CVD.49
The goal of our other secondary analyses was to determine whether a link exists between patient social factors and the likelihood of receiving screening. Neither insurance type nor race/ethnicity was associated with undergoing screening. Socioeconomic status is a challenging domain to model as it is the product of many factors not measurable in the current dataset. However, the use of both commercial payer and Medicaid insurance does provide bracketing of the socioeconomic spectrum. By restricting our study sample to subjects with continuous insurance for the entire period, we increased the internal validity of our sample and minimized misclassification of both exposure and outcome at the cost of statistical power and potential introduction of sampling bias. As a result, we are likely reporting a high-bound estimate of testing, due to censoring of families with non-continuous coverage, presumably from economic and employment instability.
Though it may be reassuring that no measurable social factors were associated with screening rates, we would not discount the importance of further research exploring the association between socioeconomic status and access to care and rates of screening. It also suggests that barriers to higher screening rates may be widespread. In previous research, significant variation in practice has been demonstrated between hospitals in multiple aspects of care of young patients with chronic (cardiac) disease.50–53 Future work should evaluate patterns of screening by practitioner, hospital, or outpatient clinic setting, and identify high- and low-performing centers, as this would help target public health policy interventions.
Conclusion
Despite several limitations, children with conditions predisposing them to early atherosclerosis were screened at a higher rate than standard-risk children, but still far below the goal of universal screening. Future research efforts should focus on specific barriers to screening for both standard- and high-risk populations and as well as the value of screening in reducing morbidity and mortality from premature cardiovascular disease.
Supplementary Material
Financial Disclosure:
The authors have no financial relationships relevant to this article to Disclose
Abbreviations:
- AAP
American Academy of Pediatrics
- AHA
American Heart Association
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- ICD
International Classification of Diseases
- NDC
National Drug Codes
- NHLBI
National Institutes of Health/National Heart, Lung, and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650–6. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC, McMahan CA, Gidding SS. Preventing Heart Disease in the 21st Century: Implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Study. Circulation 2008;117:1216–27. [DOI] [PubMed] [Google Scholar]
- 3.Malcom GT, McMahan CA, McGill J, Henry C, et al. Associations of arterial tissue lipids with coronary heart disease risk factors in young people. Atherosclerosis 2009;203:515–21. [DOI] [PubMed] [Google Scholar]
- 4.Vahamurto L, Pahkala K, Magnussen CG, et al. Coronary heart disease risk factor levels in eastern and western Finland from 1980 to 2011 in the cardiovascular risk in Young Finns study. Atherosclerosis 2019;280:92–8. [DOI] [PubMed] [Google Scholar]
- 5.Juonala M, Wu F, Sinaiko A, et al. Non-HDL Cholesterol Levels in Childhood and Carotid Intima-Media Thickness in Adulthood. Pediatrics 2020;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laitinen TT, Pahkala K, Magnussen CG, et al. Ideal Cardiovascular Health in Childhood and Cardiometabolic Outcomes in Adulthood: The Cardiovascular Risk in Young Finns Study. Circulation 2012;125:1971–8. [DOI] [PubMed] [Google Scholar]
- 7.National Cholesterol Education Program (NCEP): highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 1992;89:495–501. [PubMed] [Google Scholar]
- 8.Daniels SR, Greer FR. Lipid Screening and Cardiovascular Health in Childhood. Pediatrics 2008;122:198–208. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie SK, Murphy ECS, Ice C, et al. Universal Versus Targeted Blood Cholesterol Screening Among Youth: The CARDIAC Project. Pediatrics 2010;126:260–5. [DOI] [PubMed] [Google Scholar]
- 10.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128 Suppl 5:S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavey REW, Allada V, Daniels SR, et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation 2006;114:2710–38. [DOI] [PubMed] [Google Scholar]
- 12.Valle CW, Binns HJ, Quadri-Sheriff M, Benuck I, Patel A. Physicians’ Lack of Adherence to National Heart, Lung, and Blood Institute Guidelines for Pediatric Lipid Screening. Clin Pediatr (Phila) 2015;54:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen-Tice C, Steinberger J, Murdy K, Zierhut H. Pediatric cholesterol screening practices in 9- to 11-year-olds in a large midwestern primary care setting. J Clin Lipidol 2020;14:224–30. [DOI] [PubMed] [Google Scholar]
- 14.DeSantes K, Dodge A, Eickhoff J, Peterson AL. Improving Universal Pediatric Lipid Screening. J Pediatr 2017;188:87–90. [DOI] [PubMed] [Google Scholar]
- 15.Smith AJ, Turner EL, Kinra S. Universal Cholesterol Screening in Childhood: A Systematic Review. Acad Pediatr 2016;16:716–25. [DOI] [PubMed] [Google Scholar]
- 16.de Ferranti SD, Rodday AM, Parsons SK, et al. Cholesterol Screening and Treatment Practices and Preferences: A Survey of United States Pediatricians. J Pediatr 2017;185:99–105 e2. [DOI] [PubMed] [Google Scholar]
- 17.Dixon DB, Kornblum AP, Steffen LM, Zhou X, Steinberger J. Implementation of lipid screening guidelines in children by primary pediatric providers. J Pediatr 2014;164:572–6. [DOI] [PubMed] [Google Scholar]
- 18.Zachariah JP, McNeal CJ, Copeland LA, et al. Temporal trends in lipid screening and therapy among youth from 2002 to 2012. J Clin Lipidol 2015;9:S77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihalopoulos NL, Stipelman C, Hemond J, Brown LL, Young PC. Universal Lipid Screening in 9- to 11-Year-Olds Before and After 2011 Guidelines. Acad Pediatr 2018;18:196–9. [DOI] [PubMed] [Google Scholar]
- 20.Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics 2014;134:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornhill MH, Gibson TB, Cutler E, et al. Antibiotic Prophylaxis and Incidence of Endocarditis Before and After the 2007 AHA Recommendations. J Am Coll Cardiol 2018;72:2443–54. [DOI] [PubMed] [Google Scholar]
- 22.Janson CM, Millenson ME, Dai D, et al. Incidence of Life-Threatening Events in Children With Wolff-Parkinson-White Syndrome. Circulation 2018;138:A17031. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal A, Thombley R, Broberg CS, et al. Age- and Lesion-Related Comorbidity Burden Among US Adults With Congenital Heart Disease: A Population-Based Study. J Am Heart Assoc 2019;8:e013450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montalbano A, Rodean J, Kangas J, Lee B, Hall M. Urgent Care and Emergency Department Visits in the Pediatric Medicaid Population. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez N, Quistberg A, Vavilala MS, Jaffe KM, Rivara FP. Utilization of Mental Health Services After Mild Pediatric Traumatic Brain Injury. Pediatrics 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartz JC, Yellen E, Baker A, et al. The relationship between payer type and lipid outcomes in response to clinical lifestyle interventions in youth with dyslipidemia. BMC Pediatr 2019;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Byrne ML, Kim S, Hornik CP, et al. Effect of Obesity and Underweight Status on Perioperative Outcomes of Congenital Heart Operations in Children, Adolescents, and Young Adults: An Analysis of Data From the Society of Thoracic Surgeons Database. Circulation 2017;136:704–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation 2019;139:e603–e34. [DOI] [PubMed] [Google Scholar]
- 30.O’Byrne ML, Faerber J, Katcoff H, et al. Variation in the use of pulmonary vasodilators in children and adolescents with pulmonary hypertension: A study using data from the Marketscan® Insurance Claims Database. Pulmonary Circulation 2020;0:2045894020933083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luirink IK, Wiegman A, Kusters DM, et al. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. New England Journal of Medicine 2019;381:1547–56. [DOI] [PubMed] [Google Scholar]
- 32.Joyce N, Wellenius GA, Dore DD, Newburger JW, Zachariah JP. Patterns of Lipid Lowering Therapy among Children Ages 8–20 Years. J Pediatr 2015;167:113–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman TB, Pletcher MJ, Hulley SB. Overly aggressive new guidelines for lipid screening in children: evidence of a broken process. Pediatrics 2012;130:349–52. [DOI] [PubMed] [Google Scholar]
- 34.McCrindle BW, Kwiterovich PO, McBride PE, Daniels SR, Kavey RE. Guidelines for lipid screening in children and adolescents: bringing evidence to the debate. Pediatrics 2012;130:353–6. [DOI] [PubMed] [Google Scholar]
- 35.Force USPST. Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics 2007;120:e215–9. [DOI] [PubMed] [Google Scholar]
- 36.Gillman MW, Daniels SR. Is universal pediatric lipid screening justified? JAMA 2012;307:259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Force UPST. Screening for Lipid Disorders in Children: US Preventive Services Task Force Recommendation Statement. Pediatrics 2007;120:e215–e9. [DOI] [PubMed] [Google Scholar]
- 38.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 39.Kern L, Crow J, Williams CB, Boies E, Gahagan S, Rhee KE. Increasing Universal Lipid Screening Among 9- to 11-Year-Old Children Through a Quality Improvement Initiative. Clin Pediatr (Phila) 2017;56:640–7. [DOI] [PubMed] [Google Scholar]
- 40.Bachman RP, Schoen EJ, Stembridge A, Jurecki ER, Imagire RS. Compliance with childhood cholesterol screening among members of a prepaid health plan. Am J Dis Child 1993;147:382–5. [DOI] [PubMed] [Google Scholar]
- 41.Shamszad P, Rossano JW, Marino BS, Lowry AW, Knudson JD. Obesity and Diabetes Mellitus Adversely Affect Outcomes after Cardiac Surgery in Children’s Hospitals. Congenit Heart Dis 2016;11:409–14. [DOI] [PubMed] [Google Scholar]
- 42.Magnussen CG, Venn A, Thomson R, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol 2009;53:860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 2016;17:95–107. [DOI] [PubMed] [Google Scholar]
- 44.Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Twig G, Yaniv G, Levine H, et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N Engl J Med 2016;374:2430–40. [DOI] [PubMed] [Google Scholar]
- 46.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol 2012;27:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewitt M, Weiner SL, Simone JV. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington (DC): Institute of Medicine (US) and National Research Council (US) National Cancer Policy Board; 2003. [PubMed] [Google Scholar]
- 49.Pemberton VL, McCrindle BW, Barkin S, et al. Report of the National Heart, Lung, and Blood Institute’s Working Group on Obesity and Other Cardiovascular Risk Factors in Congenital Heart Disease. Circulation 2010;121:1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Byrne ML, Glatz AC, Faerber JA, et al. Interhospital Variation in the Costs of Pediatric/Congenital Cardiac Catheterization Laboratory Procedures: Analysis of Data From the Pediatric Health Information Systems Database. J Am Heart Assoc 2019;8:e011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Byrne ML, Glatz AC, Song L, et al. Association Between Variation in Preoperative Care Before Arterial Switch Operation and Outcomes in Patients With Transposition of the Great Arteries. Circulation 2018;138:2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glatz AC, Kennedy KF, Rome JJ, O’Byrne ML. Variations in Practice Patterns and Consistency With Published Guidelines for Balloon Aortic and Pulmonary Valvuloplasty: An Analysis of Data From the IMPACT Registry. JACC Cardiovasc Interv 2018;11:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Byrne ML, Kennedy KF, Rome JJ, Glatz AC. Variation in practice patterns in device closure of atrial septal defects and patent ductus arteriosus: An analysis of data from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2018;196:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


