Figure 3.
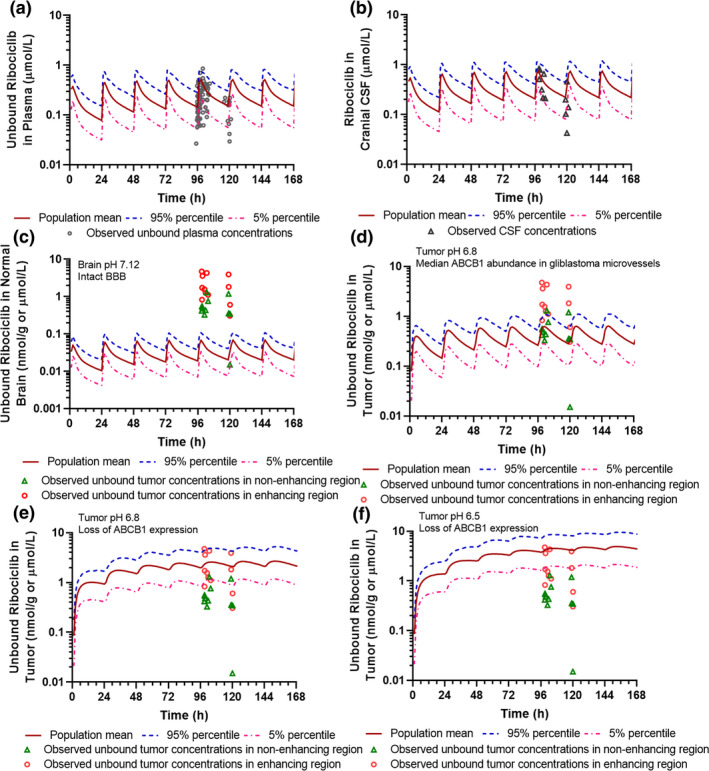
Physiologically‐based pharmacokinetic model‐simulated and clinically observed plasma and central nervous system (CNS) pharmacokinetics of ribociclib. (a) Simulated and observed unbound ribociclib plasma concentration‐time profiles. (b) Simulated and observed ribociclib cerebrospinal fluid concentration‐time profiles. (c) Simulated unbound ribociclib brain concentration‐time profiles in the human normal brain (with brain pH 7.12 and ABCB1 abundance 3.38 pmol/mg at the BBB). (d) Simulated unbound ribociclib concentration‐time profiles in brain tumors with pH 6.8 and ABCB1 abundance 0.14 pmol/mg at the BBB. (e) Simulated unbound ribociclib concentration‐time profiles in brain tumors with pH 6.8 and loss of ABCB1 expression at the BBB. (f) Simulated unbound ribociclib concentration‐time profiles in brain tumors with pH 6.5 and loss of ABCB1 expression at the BBB. Simulations of 10 trials with 10 subjects in each were performed in the Simcyp virtual cancer patient population following 5‐day ribociclib treatment at a daily oral dose of 900 mg. Observed clinical plasma and CNS pharmacokinetic data were obtained from 12 glioblastoma patients treated with ribociclib (900 mg q.d. for 5 days).
