Figure 4.
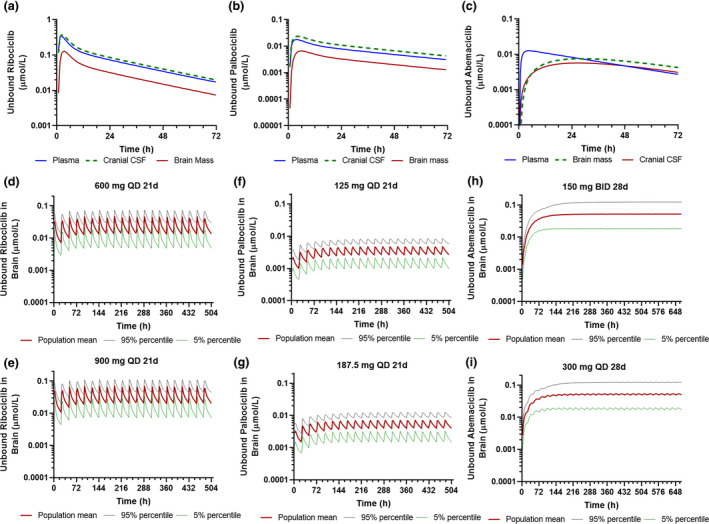
Physiologically‐based pharmacokinetic model‐simulated plasma and central nervous system pharmacokinetics of the three cyclin D‐cyclin dependent kinase 4 and 6 inhibitors following varying dosing regimens. (a) Simulated population mean plasma, CSF, and brain concentration time profiles of unbound ribociclib following a single oral dose (600 mg). (b) Simulated population mean plasma, CSF, and brain concentration time profiles of unbound palbociclib following a single oral dose (125 mg). (c) Simulated population mean plasma, CSF, and brain concentration time profiles of unbound abemaciclib following a single oral dose (150 mg). (d, e) Simulated unbound ribociclib brain concentration time profiles following the standard dosing regimen (600 mg q.d. for 3‐weeks) and modified dosing regimen (900 mg q.d. for 3 weeks). (f, g) Simulated unbound palbociclib brain concentration time profiles following the standard dosing regimen (125 mg q.d. for 3‐weeks) and modified dosing regimen (187 mg q.d. for 3 weeks). (h, i) Simulated unbound abemaciclib brain concentration time profiles following the standard dosing regimen (150 mg b.i.d. for 4‐weeks) and modified dosing regimen (300 mg q.d. for 4 weeks). Simulations of 10 trials with 10 subjects in each trial were performed in the Simcyp virtual cancer patient population.
