Figure 4: VCA tolerance is accompanied by establishment of epidermal Langerhans’ cell chimerism.
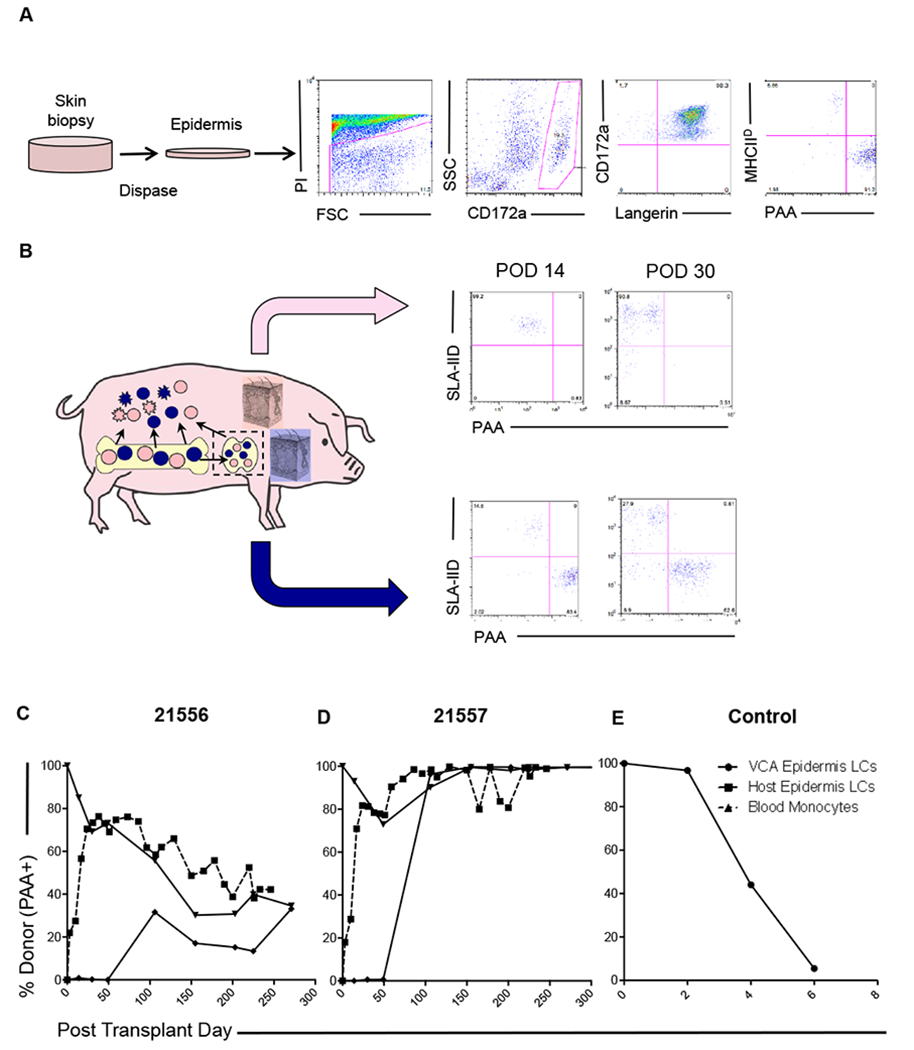
(A) Digestion of skin biopsies in dispase II facilitates independent analysis of the epidermal compartment, in which Langerhans’ cells (LCs) were identified. Chimerism was analyzed by split expression of PAA (donor) and SLA-IId (recipient). Representative data from animal 21557 day 14 is shown. (B) Chimeric VCA recipients underwent regular biopsies and parallel analysis of VCA and host skin. Recipient-derived LCs were detected early post-transplant (day 14, day 30) in VCA. Donor-derived LCs were not identified at these time points in recipient skin. Representative data from animal 21556 is shown. (C) Longitudinal analysis of epidermal LC origin demonstrated establishment of durable mixed chimerism in one recipient (21556), which equilibrated in both VCA and host skin at levels comparable to peripheral blood monocytes (dashed line). (D) In the other recipient (21557), spontaneous conversion to full donor chimerism in the myeloid lineages was detected in peripheral blood around day 50, after which time, both VCA and recipient skin epidermal LCs were found to be uniformly of donor origin. (E) In rejecting control VCAs, donor-derived LCs were progressively lost as rejection progressed, and stable chimerism was not established.
