Fig 1:
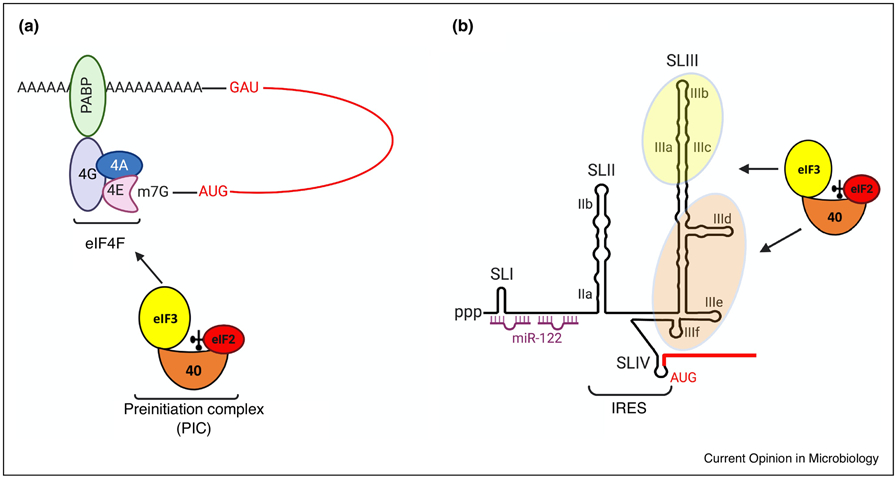
Recruitment of cellular translation machinery: (A) Translation initiation on capped, cellular mRNAs begins with the recruitment of the 43S pre-initiation complex (PIC), which contains the 40S ribosomal subunit in a complex with methionyl-initiator tRNA, GTP-bound eIF2 (depicted as a red circle bound to the 40S subunit) and eIF3 (depicted as a yellow circle bound to the 40S subunit). Capped RNAs have a 7-methyl guanosine cap at the 5’ end which interacts with the cap-binding complex (eIF4F) to recruit PICs. The eIF4F complex includes the cap binding protein eIF4E, the RNA helicase eIF4A and the scaffold protein eIF4G. eIF4G interacts with the poly A binding protein (PABP) which is usually associated with the 3’ end of polyadenylated mRNAs to promote translation initiation and ribosome recycling. The PIC scans the 5’ UTR for the start codon which is recognized through complementary base pairing with the initiator tRNA. This is followed by release of eIF2 and assembly of the 60S ribosomal subunit to form a functional 80S initiation complex that start protein synthesis. (B) HCV RNA lacks a 5’ cap structure. Instead it contains an internal ribosome entry site (IRES) that recruits PICs directly to the start codon in a sequence- and structure-dependent manner. The HCV 5’UTR is organized into four stem loop structures (SLI, SLII, SLIII and SLIV). The region spanning SLI contains two binding sites for miR-122 which promotes IRES-mediated translation. The interactions of 40S-associated eIF3 and 40S subunits with domains IIIa/b/c and IIId/e/f of the IRES, respectively, help in PIC recruitment to the start codon present in SLIV. Coding regions are indicated by red lines.
