Abstract
Rationale:
Despite anti-retroviral therapy, HIV-1 infection increases the risk of pneumonia and causes oxidative stress and defective alveolar macrophage (AM) immune function. We have previously determined that HIV-1 proteins inhibit antioxidant defenses and impair AM phagocytosis by suppressing nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Given its known effects on Nrf2, we hypothesize miR-144 mediates the HIV-1 induced suppression of Nrf2.
Methods:
Primary AMs isolated from HIV-1 transgenic (HIV-1 Tg) rats and wild type littermates (WT) as well as human monocyte-derived macrophages (MDMs) infected ex vivo with HIV-1 were used. We modulated miR-144 expression using a miR-144 mimic or an inhibitor to assay its effects on Nrf2/ARE activity and AM functions in vitro and in vivo.
Results:
MiR-144 expression was increased in AMs from HIV-1 Tg rats and in HIV-1-infected human MDMs compared to cells from WT rats and non-infected human MDMs, respectively. Increasing miR-144 with a miR-144 mimic inhibited the expression of Nrf2 and its downstream effectors in WT rat macrophages and consequently impaired their bacterial phagocytic capacity and H2O2 scavenging ability. These effects on Nrf2 expression and AM function were reversed by antagonizing miR-144 ex vivo or in the airways of HIV-1 Tg rats in vivo, but this protection was abrogated by silencing Nrf2 expression.
Conclusions:
Our results suggest that inhibiting miR-144 or interfering with its deleterious effects on Nrf2 attenuates HIV-1-mediated AM immune dysfunction and improves lung health in individuals with HIV.
Keywords: miR-144, Nrf2, alveolar macrophage, HIV-1 transgenic rat
Introduction
Infection by the human immunodeficiency virus (HIV) remains a major global threat with ~38 million individuals living with HIV worldwide.1–3 Although combination antiretroviral therapy (ART) is effective at decreasing viral load (often to undetectable levels) and slowing disease progression, individuals living with HIV remain remarkably susceptible (5-10-fold or greater) to serious pneumonias and lung injury, and pulmonary diseases remain the dominant causes of death in this vulnerable population.4, 5 Importantly, we determined that even otherwise healthy-appearing individuals living with HIV and adherent to ART have profound oxidative stress and macrophage dysfunction within their alveolar compartment,6 and recent evidence from our group and others demonstrates that HIV-1 integrates into alveolar macrophages7 and airway epithelial cells.8 Such integration in other tissues is known to lead to the chronic expression of HIV-1 viral proteins, including gp120 and Tat, which can directly induce alveolar macrophage and epithelial dysfunction.8, 9 As the alveolar macrophage is the primary innate immune cell within the alveolar space, impairments of its function by HIV-1 and/or HIV-1 viral proteins within this space likely contributes to the extraordinarily high risk of pneumonia in these individuals even when circulating virus is effectively suppressed and peripheral CD4 cell counts are in the normal range. However, the molecular mechanisms by which HIV viral proteins impair alveolar macrophage function remain only partially understood.
Using clinically relevant HIV-1 transgenic rodent models that express HIV-1 viral proteins in the alveolar space, we discovered that these proteins induce oxidative stress by inhibiting Nrf2 (Nuclear factor (erythroid-derived 2)-like 2), the master transcription factor that activates anti-oxidant and immune defenses.10, 11 Because of the important role of Nrf2 in key alveolar macrophage functions, it is critical that we understand how HIV-1 inhibits Nrf2 activity and thereby disrupts alveolar macrophage and epithelial cell function. In this study we focused on a novel mechanism by which HIV-1 infection and/or HIV-1 viral proteins within the alveolar space could inhibit Nrf2 expression and innate immune function within the alveolar macrophage. Specifically, microRNAs regulate myriad physiological mechanisms12 as they bind to the 3’ untranslated region (UTR) of their target messenger RNAs and inactivate them. Importantly, microRNA-144 (miR-144) has been identified as having protean effects that are consistent with the aforementioned effects of HIV-1 in the alveolar space. In particular, miR-144 targets the 3’-UTR of Nrf2 and inhibits Nrf2 signaling13–15 and HIV-1-induced increases in miR-144 impair alveolar epithelial barrier function.16 Therefore, we focused on miR-144 in this study and present new evidence for its role in mediating the suppression of Nrf2 and immune function in the alveolar macrophages of HIV-1 transgenic rats and possibly in human macrophages infected ex vivo with HIV. Taken together, our new experimental findings suggest that HIV-1 infection and the consequent expression of HIV-1 viral proteins in the lung induces the expression of miR-144, which inhibits Nrf2-dependent macrophage functions in the alveolar space and thereby creates a stressed microenvironment that renders these individuals susceptible to pneumonia and lung injury even when they are on effective ART.
Methods
Cell culture:
Primary alveolar macrophages (AMs) were isolated from 7-12 month old wild type Fischer 344 (WT) or HIV-1 transgenic (HIV-1 Tg) rats (Harlan Laboratories, Indianapolis, IN) and cultured in DMEM/F12 (Cellgro, Manassas, VA) with 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA) and antibiotic-antimycotic solution (Sigma-Aldrich, St. Louis, MO) at 37°C, 5% CO2. After culturing for two hours at 37°C and 5% CO2, the macrophage population was enriched by removing non-adherent cells prior to use in further experiments. A rat alveolar macrophage cell line (NR8383) was cultured in F12K with 10% FBS and antibiotic-antimycotic solution (Sigma-Aldrich).
Human MDMs:
Cells were isolated from the buffy coat of healthy human peripheral blood by Ficoll centrifugation, according to a protocol approved by Cincinnati Children’s Hospital institutional review board.10 In brief, isolated and enriched cells were cultured in RPMI-1640 with 10% FBS, 1% nonessential amino acid and 1% sodium pyruvate. After 7 days culture, cells were infected with or without HIV-1 viral particles for 4 h and then replaced with fresh growth medium for 8 days.
RNA extraction and real-time RT-PCR:
Total RNA was extracted from primary AMs using the QuickRNA kit (Zymo Research, Irvine, CA). Real-time RT-PCR was performed as previously described11 and target genes were normalized to 9S from the same RT samples.
MicroRNA isolation and analysis:
miRNA was extracted according to the mirVana miRNA Isolation Kit manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA). miRNA cDNA was synthesized using the miScript II RT Kit (Qiagen, Germantown, MD). Real-time PCR for miR-144-3p expression was performed using the Qiagen QuantiTect SYBR Green PCR Kit as previously described.16 Primers for miR-144-3p and SNORD25 were obtained from Qiagen.
Transfection of miR-144 mimic:
Primary AMs from WT rats were transfected with either miR-144-3p mimic from Qiagen (5’-UACAGUAUAGAUGAUGUACU-3’) or miRNA mimic negative control (miRNA Ng CTL, Qiagen) using Lipofectamine 3000 (Thermo Fisher Scientific). Expression of Nrf2 and its downstream effectors were assessed at 72 hours post-transfection.
miR-144 silencing in vivo:
Sixteen HIV-1 Tg rats (8 male and 8 female rats) were randomly selected to treat with 50 nM of either miRNA inhibitor negative control or miR-144-3p inhibitor (Qiagen, 5’-UACAGUAUAGAUGAUGUACU-3’) via intra-tracheal delivery three times over the course of one week as previously described.16 Phagocytic function was assessed in freshly-isolated primary AMs, as were RNA and protein expression of Nrf2 and its effectors.
Nrf2 siRNA transfection:
Primary AMs from HIV-1 Tg rats were isolated and plated at 100,000/well in 16-well chamber slides or 200,000/well in 24-well plates. The next day, AMs were transfected with 10 nM of Nrf2 stealth select RNAi (Thermo Fisher Scientific, oligo ID: RSS343557) with or without 50 nM miR-144 inhibitor using the Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). Twenty-four hours later, Nrf2 mRNA expression and phagocytic function was assessed as described below.
Nrf2 luciferase assay:
A rat macrophage cell line (NR8383, 20,000 cells/well) was cultured in 96-well plates and transfected with the Qiagen Cigna ARE reporter (which includes an inducible, ARE-responsive firefly luciferase construct and a constitutively expressed Renilla luciferase construct) and 10 nM of either miR-144 mimic or miRNA mimic control (Qiagen) using the Lipofectamine 3000 transfection reagent. Forty-eight hours later, cells were lysed and luciferase activity was quantified using a dual-luciferase reporter assay per the manufacturer’s instructions (Promega, Madison, WI). Nrf2/ARE promoter activity was expressed as a ratio of arbitrary units of firefly luciferase/Renilla luciferase activity.
Western immunoblotting:
Total proteins were isolated from the primary AMs using 2x Laemmli sample buffer (Bio-Rad Lab, Hercules, CA), electrophoresed in 4-20% polyacrylamide gels (NuSep, Germantown, MD), and transferred to a PVDF membrane. The membrane was incubated with primary antibodies against Nrf2 and NQO1 (SC-722x and SC-16464, respectively; Santa Cruz biotechnology, Heidelberg, Germany), GCLC (ab55435, Abeam, Cambridge, MA), and GAPDH for housekeeping (G9545, Sigma-Aldrich, St. Louis, MO) prior to incubation with a secondary antibody. Immuno-reactive bands were captured with the ChemiDoc XRS system (Bio-Rad).
Phagocytic assay:
Primary AMs (100,000 cells/well) were plated on 16-well chamber slides and cultured in DMEM/F12 plus 2% FBS with 10 nM of either miR-144 mimic or miRNA mimic negative control (Qiagen) for 3 days. 1 × 106 units of pHrodo Rad S. aureus BioParticle Conjugates (Thermo Fisher Scientific) were added during the final two hours of culturing. Images were captured by Olympus Fluorescent microscope and analyzed by ImageJ (NIH) as previously described.10
Hydrogen peroxide (H2O2) scavenging assay:
Primary rat AMs were cultured in 24-well plates and transfected with 10 nM of either miR-144 mimic or miRNA mimic negative control (Qiagen) for 48 hours. As previously described,10 cells were cultured with assay buffer plus 5 mU/ml of glucose oxidase (Sigma-Aldrich) at 37 °C, 5% CO2for 2 hours in the dark. The Amplex red assay kit (Thermo Fisher Scientific) was used for quantifying H2O2concentration in the culture media by measuring fluorescence at excitation 540 nm and emission 590 nm. The H2O2concentration was normalized with cells measured by MTT. The results were expressed as the percentage of H2O2 scavenging in treatment group compared to cells in control group.
Statistical analyses:
One-way ANOVA with Newman-Keuls post-test was performed for multiple comparisons and Student’s t-test was used for single comparisons using Prism (GraphPad, San Diego, CA). All data are presented as mean ± SEM. Significance was accepted at P ≤ 0.05.
Study approval:
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University. The isolation of human peripheral blood monocytes was performed at a core laboratory at Cincinnati Children’s Hospital with the approval of the Institutional Review Board at that institution. Informed consent was obtained from all participants for blood collection. All methods were performed in accordance with the relevant guidelines and regulations.
Results
HIV-1 viral proteins increased miR-144 expression in primary rat alveolar microphages and human monocyte-derived macrophages (MDMs).
We previously reported that HIV-1 transgene expression and HIV-1-infected human MDMs significantly decreased Nrf2 expression and impaired alveolar macrophage function10 and that Keap1 mRNA expression levels were stable in primary alveolar macrophages from HIV-1 transgenic rats (87.9 ± 21.7 %, n=16) compared to the cells from WT rats (100.0 ± 6.7 %, n=18). According to analysis in silico, two miR-144 binding sites are present in the Nrf2 3’-UTR. We hypothesized that HIV-1 transgene expression inhibited Nrf2 expression via alterations in miR-144 expression. As shown in Figure 1A, miR-144 expression was significantly increased 4-fold in primary alveolar microphages derived from HIV-1 Tg rats compared to cells from WT rats as well as in human MDMs infected with HIV-1 Figure 1B.
Figure 1. HIV-1 viral proteins induced miR-144 expression in macrophages.
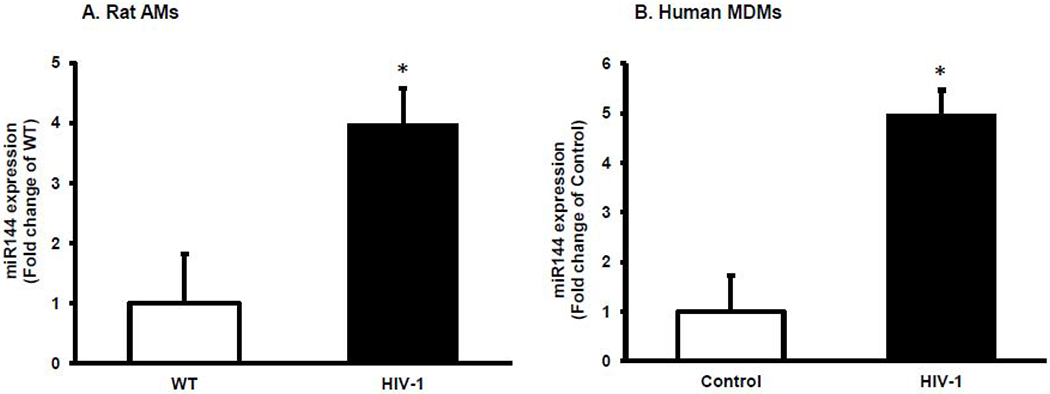
(A) miR-144 was measured by RT-qPCR in the primary alveolar macrophages derived from WT or HIV-1 Tg rats, n=7 or (B) in human MDMs infected with HIV-1 for 8 days, n=4. Student’s t-test was used for the single comparisons shown in each panel using Prism (GraphPad, San Diego, CA). In each panel, the data are shown as mean ± SEM; *p<0.05 compared to WT (panel A) and to Control (panel B).
miR-144 mimic suppressed Nrf2 and its effectors and impaired macrophage phagocytic function.
We next introduced miR-144 mimic to primary alveolar macrophages from WT rats to determine its effects on Nrf2 expression and activity. As shown in Figure 2A, increasing miR-144 significantly decreased gene expression of Nrf2 and its effectors GCLC and NQO1 (p<0.05). We confirmed these findings using a dual-luciferase assay in NR8383 cells, a rat AM cell line, and found that miR-144 mimic significantly decreased Nrf2/ARE promoter activity to around 50% of control levels (Figure 2B). Further, we determined that phagocytic function and H2O2 scavenging ability were also significantly impaired by miR-144 mimic (Figure 3A–B)
Figure 2. miR-144 mimic decreased Nrf2 activity in the alveolar macrophages in vitro.
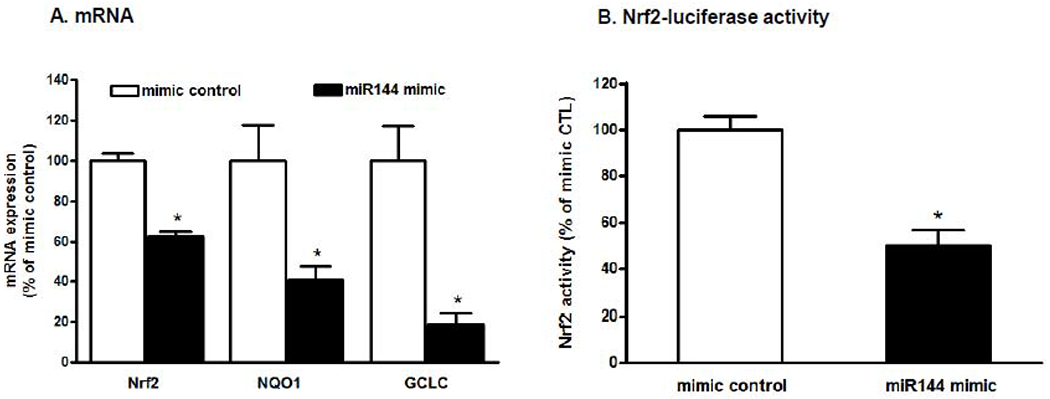
A. Primary alveolar macrophages (AM) were isolated from WT rats and transfected with 5 nM of either the miRNA mimic negative control or the miR-144 mimic for 3 days; n=3-4 wells. *P<0.05 compared to mimic negative control (p<0.05). B. NR8383 cells were transfected with 10 nM either the miRNA mimic negative control or the miR-144 mimic with the Cignal Reporter was also added. Forty-eight hours later the cells (n=12 wells in each condition) were lysed and Nrf2/ARE activity was quantified by luciferase activity. Student’s t-test was used for single comparisons using Prism (GraphPad, San Diego, CA). In each panel the data shown are the means ± SEM and *P<0.05 compared to cells treated with miRNA the mimic negative control.
Figure 3. Increasing miR-144 impairs the alveolar macrophages function in the in vitro.
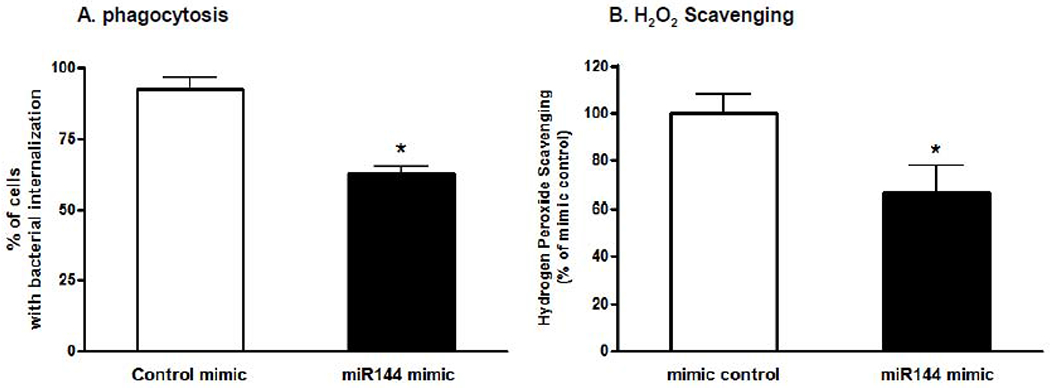
Primary alveolar macrophages (AM) were isolated from WT rats and treated with either miRNA mimic negative control or miR-144 mimic (10 nM). A. During the final 2 hours of culture, pHrodo Rad S. aureus BioParticles conjugates were added and images were captured by Olympus Fluorescent microscope and analyzed by Image J. Five images were taken from each well (150 – 160 cells per treatment); n=4-5 wells in each condition. B. H2O2 scavenging as quantified by the Amplex Red assay; n=4-5 wells in each condition. Student’s t-test was used for the single comparisons shown in each panel using Prism (GraphPad, San Diego, CA). Data shown as mean ± SEM; *P<0.05 compared to cells treated with miRNA mimic negative control.
Silencing miR-144 in vivo restored both Nrf2 expression and macrophage phagocytic function.
Because miR-144 suppressed Nrf2/ARE activity and impaired macrophage functions, we hypothesized that administration of a miR-144 inhibitor in vivo would improve viral protein-induced dysfunction in alveolar macrophages. HIV-1 Tg rats were treated with 50 nM of either miRNA inhibitor negative control or a miR-144 inhibitor intratracheally three times over the course of one week. We found that gene and protein expression of Nrf2 and its effectors NQO1 and GCLC were significantly increased in the alveolar macrophages of rats treated with the inhibitor (Figure 4A–B). Most importantly, phagocytic function in macrophages from inhibitor-treated rats was restored to WT levels (Figure 5).
Figure 4. Delivering a miR-144 inhibitor into the airway of HIV-1 transgenic rats in vivo increases the expression of Nrf2 and Nrf2-dependent antioxidants in their alveolar macrophages.
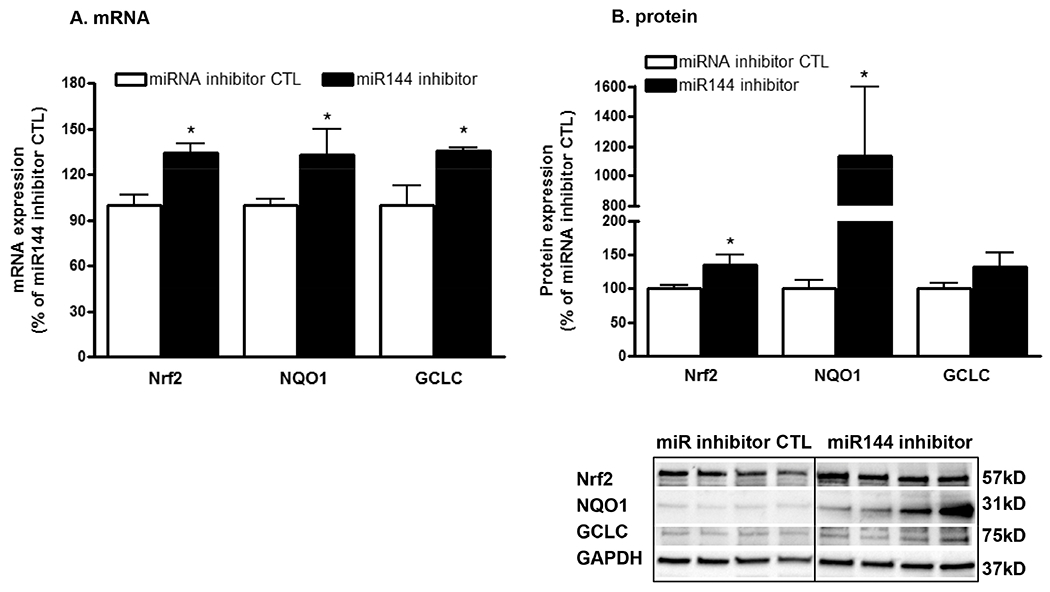
Primary alveolar macrophages (AM) were isolated from HIV-1 Tg rats treated with either the miRNA inhibitor control (CTL) or the miR-144 inhibitor (50 nM ×3) for 1 week (2 female and 2 male rats in each group). AM were then analyzed for gene expression by qRT-PCR (panel A) and protein expression by western immunoblotting (panel B). Student’s t-test was performed for single comparisons. Data are presented as mean ± SEM (n=4 rats); *P<0.05 increased compared to AM from rats treated with the miRNA inhibitor control.
Figure 5. Delivering a miR-144 inhibitor into the airway of HIV-1 transgenic rats in vivo restores the phagocytic function in their alveolar macrophages.
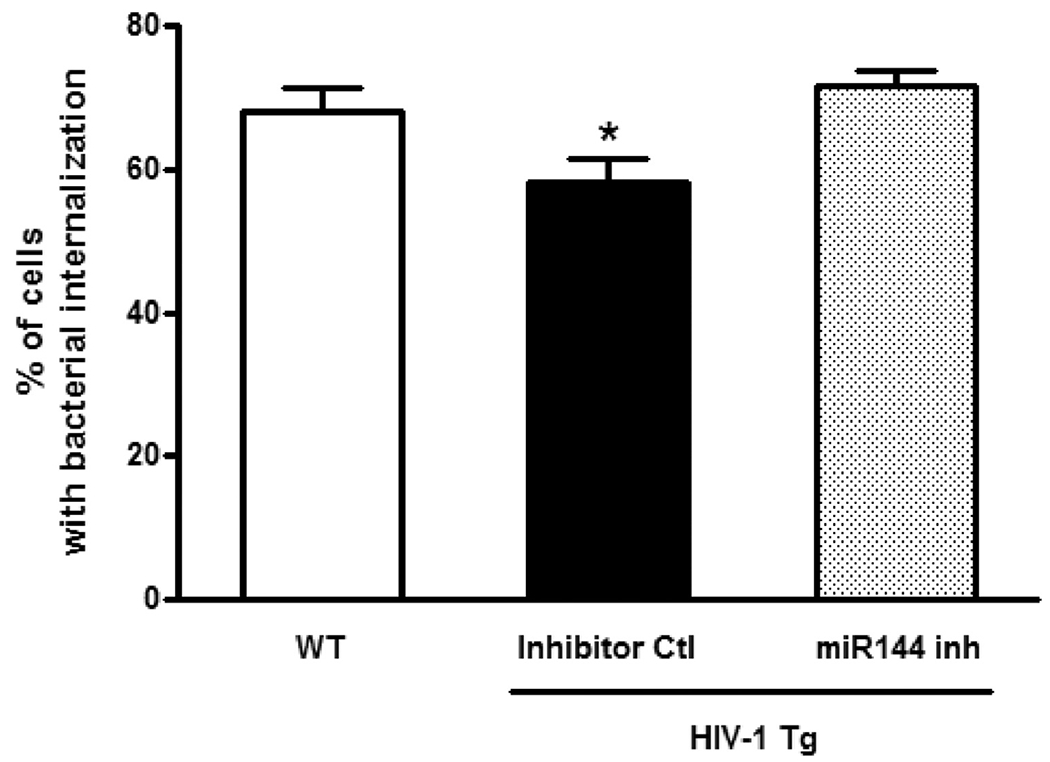
Primary alveolar macrophages (AM) were isolated from wild type (WT) rats and HIV-1 Tg rats treated with either miRNA inhibitor control or miR-144 inhibitor in vivo as in Figure 4. The AMs were then cultured for 2 hours with pHrodo Rad S. aureus BioParticle Conjugates and phagocytosis was assessed using an Olympus Fluorescent microscope and quantifying the percentage of AMs with visible bacterial internalization using ImageJ software. 10-15 images/4 rats with 30-50 cells/field were analyzed. One-way ANOVA with Newman-Keuls post-test was performed for multiple comparisons and the data are presented as mean ± SEM; *P<0.05 decreased compared to AM from WT rats and from HIV-1 Tg rats treated with the miR-144 inhibitor.
Macrophage phagocytosis is impaired by HIV-1-induced miR-144 expression.
After determining that miR-144 manipulation caused significant effects in Nrf2/ARE activity and phagocytic function, we next sought to connect the two findings through the use of Nrf2 silencing RNA. Primary alveolar macrophages from HIV-1 Tg rats were transfected ex vivo with Nrf2 silencing RNA (Nrf2 siRNA) ± miR-144 inhibitor. After 24 hours in culture, Nrf2 mRNA expression was assessed. In parallel, pHrodo-labelled S. aureus BioParticles Conjugates were added and phagocytic function was determined as described above. As expected, Nrf2 mRNA expression was significantly suppressed in cells treated with Nrf2 siRNA regardless of the presence or absence of the miR-144 inhibitor (Figure 6A). As shown in Figure 6B and consistent with the results shown in Figure 5, antagonizing miR-144 expression restored phagocytic function in alveolar macrophages from HIV-1 transgenic rats, but those effects were abrogated in cells transfected with Nrf2 siRNA. Taken together, these results are consistent with a direct mechanistic link between miR-144 and Nrf2 in the functional impairment of alveolar, macrophages during chronic exposure to HIV-1 viral proteins in vivo.
Figure 6. miR-144 inhibition restored phagocytic function in alveolar macrophages from HIV-1 Tg rats, and this protection was blocked by silencing Nrf2 expression.
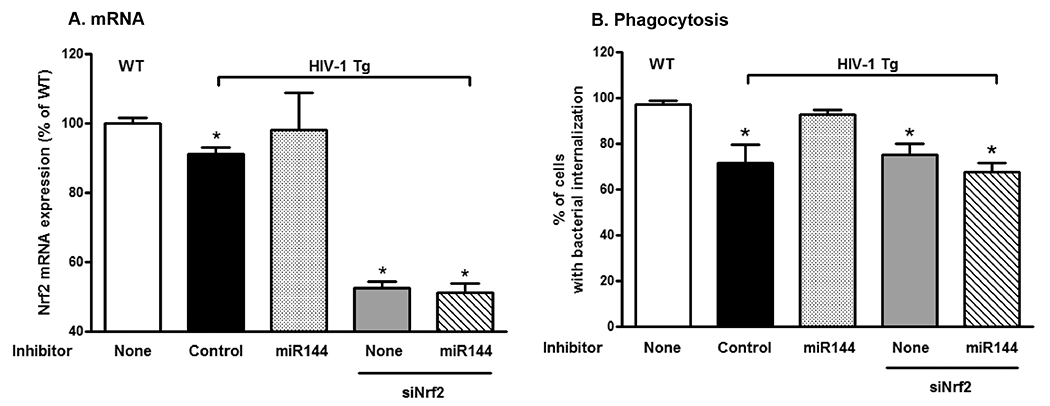
Primary alveolar macrophages (AMs) were isolated from WT and HIV-1 Tg rats. AMs from HIV-1 Tg rats were transfected ex vivo with either a control inhibitor or a miR-144 inhibitor ± Nrf2 RNA silencing (siNrf2) and then incubated with pHrodo Rad S. aureus BioParticles Conjugates as before. Twenty-four hours later, cells were extracted for analysis of Nrf2 mRNA expression (n=6 wells; panel A) and phagocytosis images (3-4 wells/treatment) were captured using an Olympus Fluorescent microscope and analyzed by Image J (150 – 200 cells per treatment; panel B). One-way ANOVA with Newman-Keuls post-tests was performed for these multiple comparisons. Data are presented as mean ± SEM; *P<0.05 decreased compared to AM from WT rats.
Discussion
In this study we determined that either HIV-1 transgene expression in vivo in rats, which causes chronic exposure of the alveolar macrophages to HIV-1 viral proteins, or direct HIV-1 infection of human monocyte-derived macrophages ex vivo, increased macrophage-specific expression of miR-144. Further, increasing miR-144 by transfecting a miR-144 mimic in primary alveolar macrophages from littermate control rats without HIV-1 transgene expression decreased Nrf2 protein levels, activity, and downstream antioxidant effector function. In parallel, miR-144 mimic decreased bacterial phagocytic capacity and hydrogen peroxide scavenging in primary alveolar macrophages. Direct increase of miR-144 in naïve macrophages recapitulated the effects of HIV-1 on these cells. In contradistinction, antagonizing miR-144 expression ex vivo in primary alveolar macrophages freshly isolated from HIV-1 transgenic rats increased the expression of Nrf2 and of downstream Nrf2-dependent antioxidants. Further, antagonizing miR-144 expression in the airways of HIV-1 transgenic rats in vivo restored alveolar macrophage phagocytic capacity. Antagonizing miR-144 expression in primary alveolar macrophages from HIV-1 transgenic rats ex vivo likewise restored their phagocytic function, but this salutary effect was abrogated by simultaneously silencing Nrf2 expression. Taken together, these experimental findings implicate the induction of miR-144 as a proximal mechanism by which HIV-1 inhibits Nrf2 expression and activity which, in turn, causes oxidative stress and immune dysfunction within the alveolar space thereby rendering individuals living with HIV-1 vulnerable to pneumonia and lung injury.
Despite the dramatic improvements in overall health and survival of people with HIV-1, even when they adhere to ART, they are at greater risk for pneumonias from diverse pathogens,17–24 and lung function is irreversibly damaged following episodes of pneumonia.25 Our group has been investigating the importance of Nrf2 suppression in HIV-1-mediated lung disease for years now. Our recent study on the effects of Nrf2 suppression in the macrophage is particularly germane here, as it highlights two key points relevant to the current investigation.10 First, it ties the effects of Nrf2 suppression directly to innate immunity. Although Nrf2 is primarily known as an antioxidant effector, its importance to macrophage innate immunity cannot be overstated.26 Second, we showed that direct infection of human monocyte-derived macrophages by HIV-1 affected Nrf2 in a similar fashion to the effects of HIV-1 viral proteins, which is important in establishing the credibility of the HIV-1 transgenic rat model we use throughout this study. Although that model is not infectious, it generates the same HIV-1 viral proteins that have been found in the alveolar space of human subjects with HIV-1. After our group noted that relatively few alveolar macrophages were infected with the virus despite the presence of global macrophage dysfunction,7 we hypothesized that the effects of HIV-1 on alveolar macrophages and epithelium may be due to viral proteins, a hypothesis that our work to date has supported.10, 11 The new findings presented in this study build on our recent manuscript in which we determined that the induction of miR-144 by HIV-1-viral proteins decreases Nrf2 expression and barrier function within the alveolar epithelium.16 Therefore, the chronic expression of HIV-1 viral proteins within the alveolar space that occurs in individuals living with HIV-1 perturbs both alveolar epithelial barrier integrity and alveolar macrophage immune function. This common mechanism, namely miR-144-mediated inhibition of Nrf2, may explain at least in part why multiple pulmonary diseases such as emphysema, pulmonary fibrosis, lung cancer and related disorders develop earlier and far more often in individuals living with HIV.27–30
In recent years, the relevance of microRNAs to human disease has made them an important topic of study.31–34 In HIV, the focus has primarily been on the effects of microRNAs in the process of HIV replication, and the manner in which microRNAs may be harnessed to control the virus.35‘36 In this study, as in our recent manuscript detailing the effects of miR-144 on alveolar epithelium,16 we highlight the effects of HIV-1 on the myriad microRNAs present in the alveolar space. Because of the complex processing they undergo, microRNAs add layers of complexity to any interaction between host and pathogen. In the case of the lung, our two studies argue that miR-144 in particular is not only enhanced by HIV-1, but also represents a proximal defect in the milieu of the alveolar space that leads directly to lung dysfunction. Further, in the case of both the macrophage (in this study) and the alveolar epithelium,16 we were able to mitigate the deleterious effects of miR-144 with an antagomir in vivo, thereby offering a compelling possibility for therapy in human subjects.
Upregulation of miR144 associated with decrease in Nrf2 expression has also been seen in other diseases such as sickle cell disease,15,37 thalassemia38 and acute myeloid leukemia.39 We focused on miR-144 based on our prior analysis in silico and our prior work on the alveolar epithelium, but our selection carries two important caveats worth mentioning here: first, several miRNAs are known to target Nrf2 based on an analysis of the miRDB (http://mirdb.org) and of the TargetScanHuman directory (http://www.targetscan.org/vert_72/). Yang et al40 reported that miR28 also targeted the Nrf2 3’UTR and reduced the Nrf2 expression in the breast cancer cell line although the complex interaction between the Nrf2 activity and cancer development and progression. Therefore, we cannot exclude the possibility that other miRNAs contribute to the pathophysiological effects of HIV-1 viral proteins on Nrf2 and its effectors. However, at least in the experimental HIV-1 transgenic rat model employed in this study, specifically increasing miR-144 by transfecting a mimic recapitulates the deleterious effects of HIV-1 viral proteins on Nrf2 expression and phagocytic function in the alveolar macrophage and antagonizing miR-144 reverses these effects. Importantly, although we could not perform such parallel mechanistic studies in human cells, we determined that direct infection of human macrophages with HIV-1 induced the expression of miR-144, providing important albeit circumstantial evidence that this pathophysiological sequence is relevant in individuals living with chronic HIV. Interestingly, miR-144 appears to mediate influenza-induced lung injury as ablating miR-144 in vivo attenuates influenza replication and lung injury in mice.41 However, to date, studies connecting HIV-1 and miR-144 have been few and far between, including one recent clinical investigation42 and our own recent paper on HIV-1-induced epithelial dysfunction.16
In summary, we determined that HIV-1 infection of human macrophages, or the chronic expression of HIV-1 viral proteins within the alveolar space in a transgenic animal model, induces miR-144. In the experimental model we identified that miR-144 in turn inhibits the expression and functions of Nrf2 and thereby dampens bacterial phagocytic capacity. Further, antagonizing miR-144 in the airways of HIV-1 transgenic animals in vivo restores Nrf2 expression and function within the alveolar macrophage and reverses their phagocytic dysfunction. These findings provide new insights into why individuals living with HIV are at increased risk for pneumonia and other lung diseases despite effective viral suppression and apparent immune reconstitution with ART. Further, they suggest that targeting miR-144 and/or Nrf2 within the airway could enhance lung health in this vulnerable population.
Acknowledgments
We thank S. Todd Mills and Lingmei Ding for their excellent technical support.
Funding sources: Funding for this work was provided by R01HL125042 (DMG), R01AI150475 (PS) and K08 AA024512 (BSS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interests.
References
- 1.Beck JM. Abnormalities in host defense associated with HIV infection. Clin Chest Med 2013;34:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staitieh B, Guidot DM. Noninfectious pulmonary complications of human immunodeficiency virus infection. The American journal of the medical sciences 2014;348:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Global HIV & AIDS statistics - 2019 fact sheet. 2019. [Google Scholar]
- 4.Afessa B, Green W, Chiao J, et al. Pulmonary complications of HIV infection: autopsy findings. Chest 1998;113:1225–9. [DOI] [PubMed] [Google Scholar]
- 5.Hung CC, Chang SC. Impact of highly active antiretroviral therapy on incidence and management of human immunodeficiency virus-related opportunistic infections. J Antimicrob Chemother 2004;54:849–53. [DOI] [PubMed] [Google Scholar]
- 6.Cribbs SK, Guidot DM, Martin GS, et al. Anti-retroviral therapy is associated with decreased alveolar glutathione levels even in healthy HIV-infected individuals. PloS one 2014;9:e88630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cribbs SK, Lennox J, Caliendo AM, et al. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS research and human retroviruses 2015;31:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi PC, Raynor R, Fan X, et al. HIV-1-transgene expression in rats decreases alveolar macrophage zinc levels and phagocytosis. American journal of respiratory cell and molecular biology 2008;39:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassiter C, Fan X, Joshi PC, et al. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS research and therapy 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staitieh BS, Ding L, Neveu WA, et al. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. Journal of leukocyte biology 2017;102:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan X, Staitieh BS, Jensen JS, et al. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am J Physiol Lung Cell Mol Physiol 2013;305:L267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev 2011;91:827–87. [DOI] [PubMed] [Google Scholar]
- 13.Narasimhan M, Patel D, Vedpathak D, et al. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PloS one 2012;7:e51111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Yu S, Zhang C, et al. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med 2015;88:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangokoya C, Telen MJ, Chi JT. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010;116:4338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kukoyi AT, Fan X, Staitieh BS, et al. MiR-144 mediates Nrf2 inhibition and alveolar epithelial dysfunction in HIV-1 transgenic rats. Am J Physiol Cell Physiol 2019;317:C390–C7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujitani S, Sun HY, Yu VL, et al. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest 2011;139:909–19. [DOI] [PubMed] [Google Scholar]
- 18.Afessa B, Green B. Clinical course, prognostic factors, and outcome prediction for HIV patients in the ICU. The PIP (Pulmonary complications, ICU support, and prognostic factors in hospitalized patients with HIV) study. Chest 2000;118:138–45. [DOI] [PubMed] [Google Scholar]
- 19.Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest 2000;117:1017–22. [DOI] [PubMed] [Google Scholar]
- 20.Shankar EM, Kumarasamy N, Rajan R, et al. Aspergillus fumigatus, Pneumocystis jiroveci, Klebsiella pneumoniae & Mycoplasma fermentans co-infection in a HIV infected patient with respiratory conditions from Southern India. Indian J Med Res 2006;123:181–4. [PubMed] [Google Scholar]
- 21.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med 2001;161:441–6. [DOI] [PubMed] [Google Scholar]
- 22.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med 1995;333:845–51. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CF Jr., Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 2007;5:298–308. [DOI] [PubMed] [Google Scholar]
- 24.Staitieh BS, Egea EE, Guidot DM. Pulmonary Innate Immune Dysfunction in Human Immunodeficiency Virus. American journal of respiratory cell and molecular biology 2017;56:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris AM, Huang L, Bacchetti P, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 2000;162:612–6. [DOI] [PubMed] [Google Scholar]
- 26.Staitieh BS, Fan X, Neveu W, et al. Nrf2 regulates PU.1 expression and activity in the alveolar macrophage. Am J Physiol Lung Cell Mol Physiol 2015;308:L1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–33. [DOI] [PubMed] [Google Scholar]
- 28.Crothers K Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med 2007;28:575–87, vi. [DOI] [PubMed] [Google Scholar]
- 29.Gingo MR, Morris A, Crothers K. Human immunodeficiency virus-associated obstructive lung diseases. Clin Chest Med 2013;34:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–72. [DOI] [PubMed] [Google Scholar]
- 31.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature reviews Drug discovery 2017;16:203–22. [DOI] [PubMed] [Google Scholar]
- 32.Maltby S, Plank M, Tay HL, et al. Targeting MicroRNA Function in Respiratory Diseases: Mini-Review. Frontiers in physiology 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown D, Rahman M, Nana-Sinkam SP. MicroRNAs in respiratory disease. A clinician’s overview. Annals of the American Thoracic Society 2014;11:1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferruelo A, Penuelas O, Lorente JA. MicroRNAs as biomarkers of acute lung injury. Annals of translational medicine 2018;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klase Z, Houzet L, Jeang KT. MicroRNAs and HIV-1: complex interactions. J Biol Chem 2012;287:40884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasubramaniam M, Pandhare J, Dash C. Are microRNAs Important Players in HIV-1 Infection? An Update. Viruses 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Zhu X, Ward CM, et al. MIR-144-mediated NRF2 gene silencing inhibits fetal hemoglobin expression in sickle cell disease. Exp Hematol 2019;70:85–96 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinoun K, Sathirapongsasuti N, Paiboonsukwong K, et al. miR-144 regulates oxidative stress tolerance of thalassemic erythroid cell via targeting NRF2. Ann Hematol 2019;98:2045–52. [DOI] [PubMed] [Google Scholar]
- 39.Sun X, Liu D, Xue Y, et al. Enforced miR-144–3p Expression as a Non-Invasive Biomarker for the Acute Myeloid Leukemia Patients Mainly by Targeting NRF2. Clin Lab 2017;63:679–87. [DOI] [PubMed] [Google Scholar]
- 40.Yang M, Yao Y, Eades G, et al. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res Treat 2011;129:983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberger CM, Podyminogin RL, Diercks AH, et al. miR-144 attenuates the host response to influenza virus by targeting the TRAF6-IRF7 signaling axis. PLoS Pathog 2017;13:e1006305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson SM, Naidoo RN, Pillay Y, et al. HIV induced nitric oxide and lipid peroxidation, influences neonatal birthweight in a South African population. Environment international 2018;121:1–12. [DOI] [PubMed] [Google Scholar]


