Figure 6.
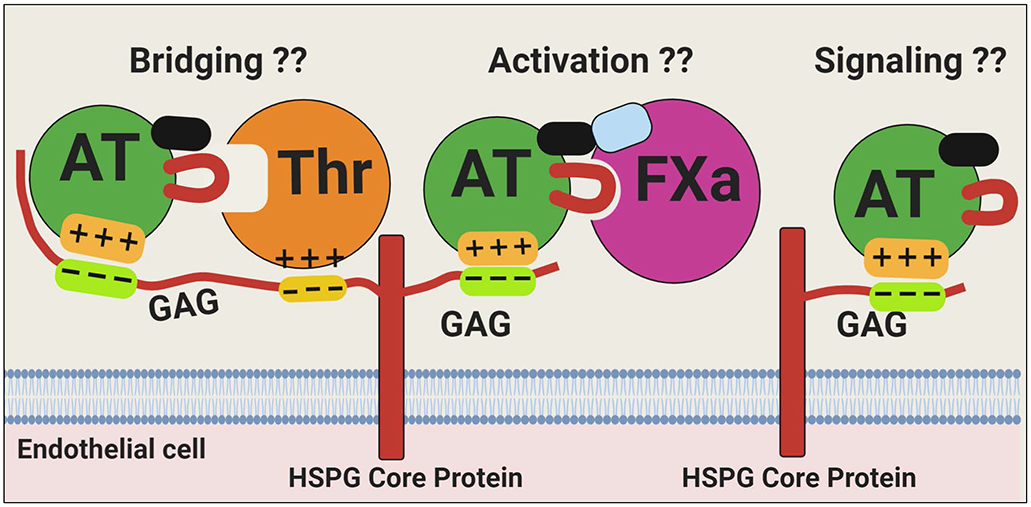
Hypothetical model of the interaction of AT with different vascular GAGs. Vascular HSPGs containing GAGs with different chain-lengths can bind D-helix of AT to elicit intracellular signaling responses and/or promote the serpin inhibition of coagulation proteases by a conformational activation (FXa) or by a template (thrombin) mechanism. These mechanistic concepts have been firmly established in cellular and in in vitro assays using therapeutic heparins. However, the relevance/significance of AT interaction with vascular GAGs to physiological functions (signaling or protease inhibitory function) of AT remains unknown (??) and requires further investigation. Figure was prepared by software provided by Biorender.com.
