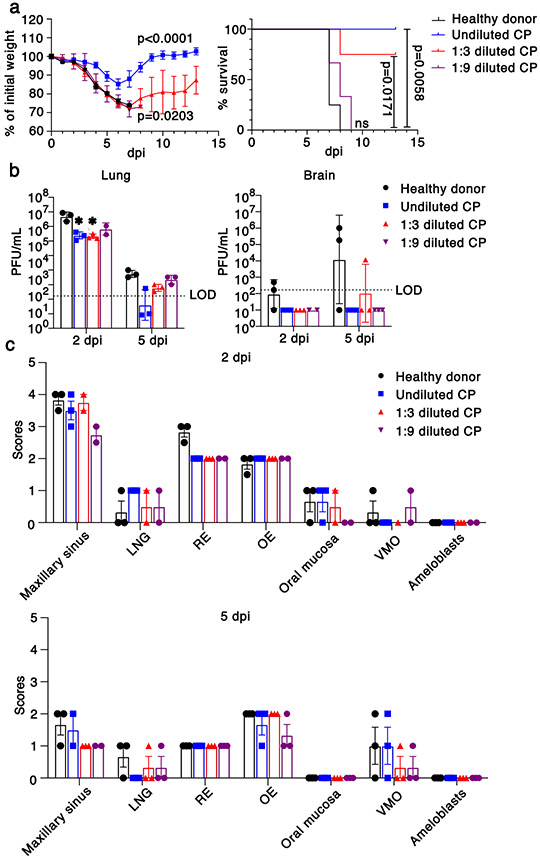
Fig. 3. Effects of convalescent plasma (CP) on outcomes.
a. Percentage of initial weight (left panel) and survival (right panel) of K18-hACE2 mice receiving control serum (n=4 mice, black), undiluted (n=4 mice, blue), 1:3 dilution (n=4 mice, red) and 1:9 dilution (n=3 mice, purple) human CP at 24 hours prior to challenge with 105 PFU SARS-CoV-2. Data are from two independent experiments. ANOVA and 2-tailed Student’s t tests without adjustments (weight change) and log-rank (Mantel-Cox) tests (survival) were used to analyze these data. b. Viral titers of CP-treated mice in the lungs (left panel) and brains (right panel) at 2 and 5 dpi. n=3 except for 2 dpi, 1:9 dilution (n=2) mice, 1 independent experiment. LOD=limit of detection. 2-tailed Student’s t tests without adjustments were used to analyze these data. * P=0.0455, control vs. undiluted CP; P=0.0443 control vs. 1:3 diluted CP. c. Scores of N protein immunostaining in CP-treated mice in the nasal cavity at 2 (upper panel) and 5 (lower panel) dpi. 0 – none; 1 - rare <1%; 2 - multifocal or localized <33% cells; 3 - multifocal, coalescing, 33-66%; 4 - extensive >67%. Two sections of each sinonasal cavity from three mice per group were evaluated. Data are shown as mean±SEM. LNG-lateral nasal gland, RE-respiratory epithelium, OE-olfactory epithelium, VMO-vomeronasal organ.