Abstract
The lamina cribrosa (LC) region of the optic nerve head (ONH) is considered a primary site for glaucomatous damage. In humans, biology of this region reflects complex interactions between retinal ganglion cell (RGC) axons and other resident ONH cell-types including astrocytes, lamina cribrosa cells, microglia and oligodendrocytes, as well as ONH microvasculature and collagenous LC beams. However, species differences in the microanatomy of this region could profoundly impact efforts to model glaucoma pathobiology in a research setting. In this study, we characterized resident cell-types, ECM composition and ultrastructure in relation to microanatomy of the ONH in adult domestic cats (Felis catus). Longitudinal and transverse cryosections of ONH tissues were immunolabeled with astrocyte, microglia/macrophage, oligodendrocyte, LC cell and vascular endothelial cell markers. Collagen fiber structure of the LC was visualized by second harmonic generation (SHG) with multiphoton microscopy. Fibrous astrocytes form glial fibrillary acidic protein (GFAP)-positive glial columns in the pre-laminar region, and cover the collagenous plates of the LC region in lamellae oriented perpendicular to the axons. GFAP-negative and alpha-smooth muscle actin-positive LC cells were identified in the feline ONH. IBA-1 positive immune cells and von Willebrand factor-positive blood vessel endothelial cells are also identifiable throughout the feline ONH. As in humans, myelination commences with a population of oligodendrocytes in the retro-laminar region of the feline ONH. Transmission Electron microscopy confirmed the presence of capillaries and LC cells that extend thin processes in the core of the collagenous LC beams. In conclusion, the feline ONH closely recapitulates the complexity of the ONH of humans and non-human primates, with diverse ONH cell-types and a robust collagenous LC, within the beams of which, LC cells and capillaries reside. Thus, studies in a feline inherited glaucoma model have the potential to play a key role in enhancing our understanding of ONH cellular and molecular processes in glaucomatous optic neuropathy.
Keywords: Glaucoma, Optic nerve head, Lamina cribrosa, Cat, Lamina cribrosa cell, Astrocyte, Collagen, Extracellular matrix
1. Introduction
Glaucoma is a leading cause of irreversible vision loss and is estimated to affect more than 70 million people worldwide by 2020 (Tham et al., 2014). It is characterized by an optic neuropathy representing progressive optic nerve degeneration and loss of retinal ganglion cells (RGCs) (Weinreb et al., 2014). Elevated intraocular pressure (IOP) is the most consistent risk factor in the development and progression of glaucomatous optic neuropathies (Leske et al., 2007). One of the key hallmarks of glaucomatous optic neuropathy is progressive excavation and remodeling (clinically recognized as “cupping”) of the optic nerve head (ONH), where RGC axons converge and exit the eye (Burgoyne, 2015; Yang et al., 2017). The human ONH possesses a lamina cribrosa (LC), that is composed of multi-layered collagenous beams with numerous pores through which RGC axon bundles pass (Anderson, 1969; Downs and Girkin, 2017; Jonas et al., 1991; Radius and Gonzales, 1981). Deformation and remodeling of the LC is a consistent feature of human glaucoma (Quigley and Green, 1979), and the ONH is considered an initial site for axonal damage in glaucoma (Howell et al., 2007). However, ONH biology is complex, with intimate interactions between RGC axons and resident ONH cells including different types of glial cells (astrocytes, microglia and oligodendrocytes) and lamina cribrosa cells in addition to ONH microvasculature, myelination and extracellular matrix (ECM) components including those of the robust LC tissue. These relationships are critical to RGC axonal health, and dysfunction of these components lead to optic nerve degeneration under pathological conditions in glaucoma.
Within the human and non-human primate ONH, are three distinct sub-regions: pre-laminar (PL); lamina cribrosa (LC), and retro-laminar (RL) (Anderson, 1969; Minckler et al., 1976; Balaratnasingam et al., 2009), each with distinct functions and resident cell populations and differing patterns of axonal cytoskeletal protein expression (Balaratnasingam et al., 2009; Kang and Yu, 2015). It has been proposed that this marked heterogeneity between ONH sub-regions in their ECM composition (Hernandez et al., 1986; Goldbaum et al., 1989; Morrison et al., 1995), cell-type, microvasculature (Kang et al., 2018), myelination (Anderson, 1969; Elkington et al., 1990), mitochondrial activity (Bristow et al., 2002; Barron et al., 2004) and axonal structural components (Kang et al., 2014), may play a key role in determining regional vulnerability to injury and disease. In particular, a growing body of evidence supports the involvement of multiple resident cell types of the ONH in glaucoma pathobiology (Nickells et al., 2012; Williams et al., 2017).
Animal models have greatly advanced our understanding of pathobiological processes in glaucoma. However, species differences in microanatomy of the ONH could profoundly impact the relevance of animal models of human glaucomatous optic neuropathy. For example, the ONHs of widely used rodent models such as mice and rats lack a collagenous LC (May and Lütjen-Drecoll, 2002; Morrison et al., 1995).
Domestic cats (Felis Catus) have been widely used in studies that have provided a foundation for our understanding of the mammalian visual system and its development (Ng and Stone, 1982; Sherman and Spear, 1982). Specific to glaucoma, feline models have been used to study the effect of experimental optic nerve crush or acute and chronic induction of high IOP on RGC structure and function, axoplasmic flow and axon degeneration (Chen and Weber, 2001; Radius and Bade, 1981, 1982a; Shou et al., 2003; Siliprandi et al., 1988). In addition, spontaneous glaucoma in cats is well documented in the veterinary literature (McLellan and Teixeira, 2015). A viable colony of cats with recessively inherited feline congenital glaucoma (FCG) has been established (Kuehn et al., 2016; Rutz-Mendicino et al., 2011), and represents an ortholog of human glaucoma due to mutation in LTBP2 (GLC3C: OMIM#613086) (Ali et al., 2009; Narooie-Nejad et al., 2009). Elevation of IOP is recognized early in life in this spontaneous feline model (Adelman et al., 2018) but glaucomatous optic nerve damage is slowly progressive, as in many forms of glaucoma in humans. Although the presence of a collagenous LC in cats has previously been documented (Radius and Bade, 1982b), there is a lack of published studies that characterize and quantify diverse resident ONH cell-types, and their relation to the complex ONH microanatomy in cats. Cats with FCG have the potential to serve as a highly translationally-relevant animal model of glaucoma, with large eyes comparable in size to those of humans, and a well-developed, well-characterized visual system. The aim of this study was to address critical gaps in knowledge regarding microanatomy and sub-regional heterogeneity in resident cell-types of the feline ONH. Here we highlight the translational relevance of feline models by clearly demonstrating that the feline ONH resembles that of humans and non-human primates in terms of resident cell-types, sub-regional heterogeneity in cell populations, ECM composition, ultrastructure, and LC microstructure. We have identified LC cells in the ONH in situ, for the first time to our knowledge, in a non-primate species that also provides a spontaneous model of glaucoma.
2. Materials and methods
2.1. Tissues, tissue preparation and histology for light microscopy
Eleven archived, fixed eyes from 10 normal young adult domestic cats, 1–2 years of age, were studied. Eyes had been enucleated previously, immediately following humane euthanasia for reasons unrelated to the present study, and fixed in 4% paraformaldehyde (PFA) in 0.01M PBS (10mM phosphate, 15.4mM sodium chloride, pH 7.4) overnight at 4 °C, and then transferred into 0.01M PBS and stored at 4°C until use. The ONH was trephined using a 4mm-biopsy punch.
For general histology, the ONH tissues from two of these feline eyes were dehydrated, paraffin-embedded and sectioned to a thickness of 5μm. The sections were de-paraffinized with xylenes and then rehydrated with a series of ethanol and stained with either hematoxylin and eosin (H&E), Masson’s trichrome or picrosirius red prior to examination by light microscopy.
2.2. Immunofluorescence
Immunofluorescence labeling was performed as previously described with a minor modification (Snyder et al., 2019). Briefly, 4% PFA-fixed ONH tissues from 9 feline eyes were cryoprotected in a series of sucrose from 10% – 30% w/v, embedded in OCT compound (Tissue-Tek, Sakura, Japan) and sectioned at 10 μm thickness. Sections were permeabilized and blocked in a blocking solution [10% normal donkey serum, 2% bovine serum albumin (BSA) and 0.2% Triton X-100 in 0.01M PBS] for 1 hr at room temperature, and then incubated with primary antibodies recognizing glial fibrillary acidic protein (GFAP, Invitrogen, catalog# 13-0300, at 1:2000 dilution); SRY-Box transcription factor 9 (SOX9, abcam, catalog# ab185966, at 1:1000 dilution); alpha-smooth muscle actin [IA4] (α-SMA, Novus Biologicals, catalog# NBP2-33006, at 1:400 dilution); von Willebrand Factor (vWF, Novus Biologicals, catalog# NB600-586, at 1:1000 dilution); ionized calcium-binding adapter molecule-1 (IBA-1, abcam, catalog# ab178846, at 1:2000 dilution); Neurofilament heavy chain (NF-H, Novus Biologicals, catalog# NB300-217, at 1:2000 dilution); myelin basic protein (MBP, Novus Biologicals, catalog# NB600-717, at 1:50 dilution); oligodendrocyte transcription factor 2 (OLIG2, Millipore, catalog# AB9610, at 1:400 dilution); adenomatous polyposis coli [CC-1] (APC, Novus Biologicals, catalog# NB600-1021, at 1:1000 dilution); collagen I (Novus Biologicals, catalog# NB600-450, at 1:500 dilution); collagen III (Novus Biologicals, catalog# NB600-594, at 1:200 dilution); collagen IV (Novus Biologicals, catalog#: NB120-6586, at 1:200 dilution); collagen VI (Novus Biologicals, catalog# NB120-6588, at 1:200 dilution); Elastin (Novus Biologicals, catalog# NB100-2076, at 1:50 dilution), and / or rabbit IgG isotype control (Novus Biologicals, catalog# NBP2-24891, at 1:100 dilution). Primary antibodies were detected by appropriate Alexa® 488, 568 and 647 conjugated secondary antibodies at 1:500 dilution in 0.01M PBS and nuclei counterstained with DAPI (1:10000 dilution in 0.01M PBS) for 1 hr at room temperature. For negative controls, sections were prepared by omission of primary antibodies, followed by incubation with secondary antibodies. For immunolabeling of collagens, a rabbit isotype control antibody was used. Images were captured either using a Leica SP8 confocal microscope with LAS X software (ver.3.1) or a Zeiss Axio Imager Z.2 fluorescence microscope with Zen Pro software (ver 2.3).
2.3. Transmission electron microscopy (TEM)
Transmission electron microscopy was performed as previously described (Scott et al., 2016). Briefly, optic nerve head tissues were fixed in 4% paraformaldehyde (PFA) overnight and post-fixed in 2.5% glutaraldehyde before osmication in 1% osmium tetroxide and embedding in acrylic resin. Thin sections (approximately 90 nm) were cut, mounted on 200 mesh copper grids, stained with 5% methanolic uranyl acetate and Reynolds’ lead citrate, and then examined and photographed using a Philips 410 transmission electron microscope (Philips Medical Systems, Andover, MA).
2.4. Second harmonic generation (SHG)
A custom multiphoton workstation at UW-Madison’s Laboratory for Optical and Computational Instrumentation (LOCI) was used to image tissue slides with second harmonic generation (SHG) using a TE300 inverted microscope (Nikon, Tokyo, Japan) equipped with a CFI Plan Apo 40X (N.A = 1.15; Nikon) water immersion objective lens and a mode-locked Ti: Sapphire laser (Mai Tai Deepsee; Spectra Physics, Mountain View, CA). The excitation wavelength was tuned to 890 nm; a 445 nm ± 25 nm narrow bandpass emission filter (Semrock) was used to detect the SHG signal of collagen in the backscattered mode using a H7422P-40 GaAsP photon counting PMT (Hamamatsu Photonics, Hamamatsu City, Japan). Images were acquired using WiscScan (LOCI, University of Wisconsin, Madison). A total of 10–15 optical sections were acquired, and image stacks were processed using Fiji (Schindelin et al., 2012) to generate maximum intensity Z projections.
2.5. ONH cell quantification
For ONH cell quantification and image analyses using Fiji / ImageJ, images of immunolabeled 10μm thick ONH tissue sections from 4 cats were acquired using a Zeiss Axio Imager Z.2 fluorescence microscope with 20x objective lens. Multiple images were stitched to generate a tile-scan of the entire ONH tissue section using Zen Pro software (ver 2.3). The area of each ONH sub-region in these tiled images were delineated manually (Fig. 4) as previously described (Balaratnasingam et al., 2014). Differential interference contrast (DIC) was used to identify the location of the collagenous LC. Total numbers of nuclei counterstained with DAPI, GFAP+SOX9+ astrocytes, IBA-1+ microglia and OLIG2+ oligodendrocyte lineage cells as well as α-SMA+GFAP− LC cells in each ONH sub-region were counted. For each cat, quantification of each ONH cell type was conducted in 3 representative sections for each of the ONH sub-regions, averaging values calculated for each subregion. Data are presented as mean densities of each cell-type, as well as proportion of each cell type relative to the total number of DAPI+ cells, calculated for each ONH sub-region.
Fig. 4.

Sub-regions of feline optic nerve head. Representative photomicrographs of a longitudinal section of normal feline optic nerve head (ONH) immunolabeled for myelin basic protein (MBP; A), neurofilament (NF; B) and nuclei counterstained with DAPI (C), and imaged with differential interference contrast (DIC) for visualization of collagenous lamina cribrosa (D). In the multichannel merged image (E), three distinct sub-regions are: pre-laminar (PL); lamina cribrosa (LC), and retro-laminar (RL). The retinal nerve fiber layer (RNFL) is also labeled for reference. Note that immediately posterior to the LC, which is delineated by white broken lines, myelination of optic nerve axons commences in the RL region of the feline ONH. Scale bars = 200μm.
2.6. Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (ver 7.0; GraphPad Software, San Diego, CA, USA). Quantitative values were compared between groups using unpaired student t-test or ANOVA with Tukey’s multiple comparison test, with P < 0.05 considered significant.
3. Results
3–1. Robust collagenous lamina cribrosa (LC) in the feline ONH
Histochemical staining of feline ONH tissue sections confirmed the presence of collagenous LC in the feline ONH, spanning the scleral canal (Fig. 1A–L). The feline LC was further characterized by immunolabeling as being comprised of predominantly collagen type I (Fig. 2A) while second harmonic generation (SHG) microscopy of transverse (coronal) sections of the ONH at the level of LC (Fig. 2B) further illustrated its complex microstructure, comprising stacked, collagen-rich lamellar beams interspersed with numerous pores. In this scope of this study only qualitative assessment of the SHG data was conducted but key features of the collagen fibers can be observed such as distinct wavy patterns known as fibrillar crimps (Franchi et al., 2008; Jan et al., 2017) (Fig. 2C).
Fig. 1.
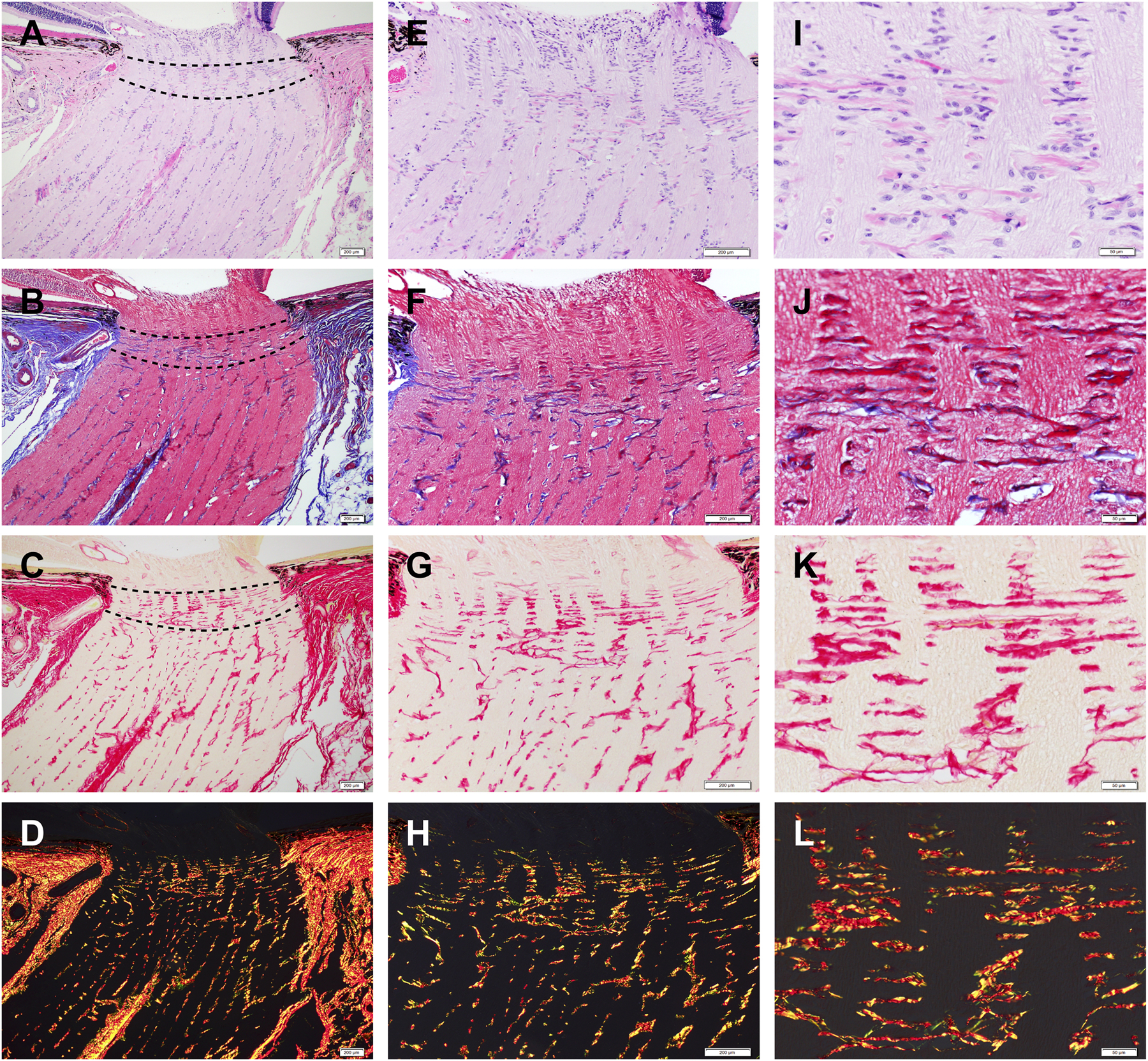
Representative histology images of longitudinal sections of feline optic nerve head (ONH) stained with hematoxylin and eosin (H&E) (A, E, I); Masson’s trichrome (B, F, J); picrosirius red staining using bright field (C, G, K) and polarization microscopy (D, H, L), to highlight the connective tissue composition in the feline ONH and horizontally aligned collagenous beams in the LC (delineated by broken lines in A, B, C ). Scale bars = 200 μm (A-H), 50 μm (I-L).
Fig. 2.
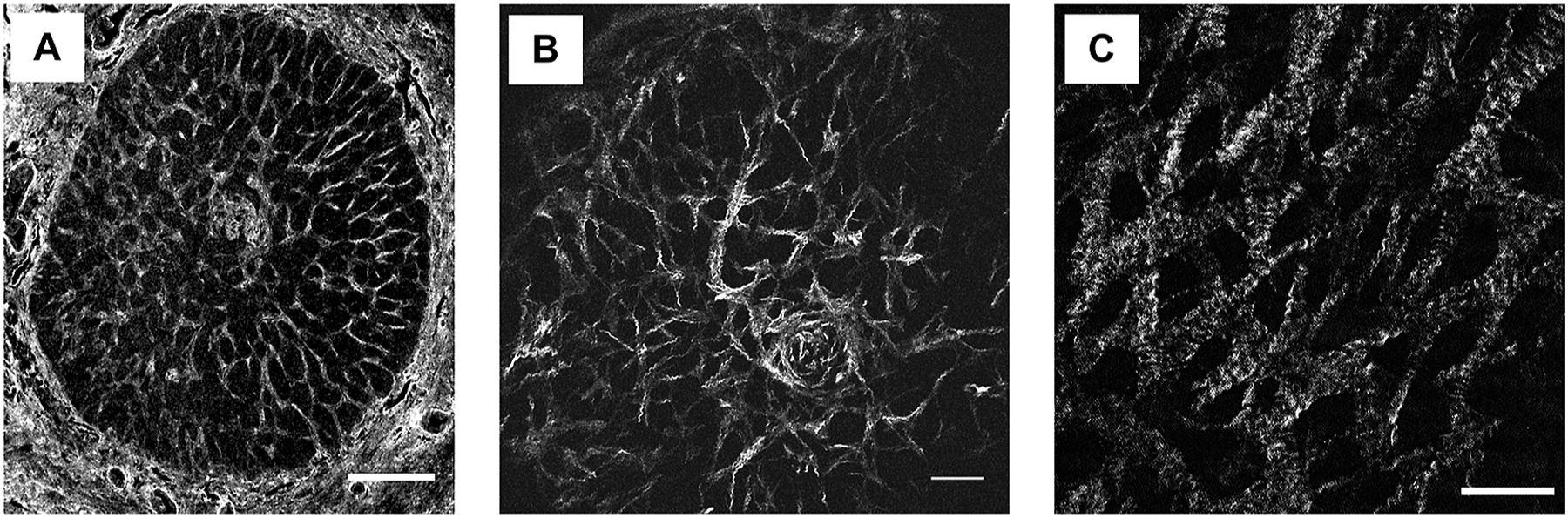
Feline ONH possesses a robust collagenous LC. (A) Immunofluorescent labeling of collagen type 1 on transverse optic nerve head (ONH) section at the level of the lamina cribrosa (LC) demonstrating a connective tissue network comprised of many interconnecting collagen beams. (B) Second harmonic generation (SHG) showing the complex microstructure of collagenous beams of the LC and, on higher magnification (C) key SHG features of the collagen fibers include a wavy, banded structure consistent with fibrillar crimps. Scale bars = 200μm (A), 100μm (B) 50μm (C).
3–2. ECM composition of the feline LC
The expression patterns of collagen types I, III, IV and VI as well as elastin in the feline ONH were examined. In addition to collagen type I expression in the feline LC as a major constituent of the LC beams (as shown in Fig. 2A), immunofluorescence labeling demonstrated collagens type III and VI expression patterns consistent with their presence within the LC beams (Fig. 3A, B), and collagen type IV (basement membrane) expression appeared to be associated with blood vessels and at the interface between the LC beams and astrocytes (Fig. 3C), with no detectable signal in negative control sections (Fig. 3D). Elastin expression in the LC was also identified by immunolabeling on feline ONH transverse sections, which demonstrated straight elastic fibers originating from the elastic fiber network in the peripapillary sclera. (Fig. 3E).
Fig. 3.
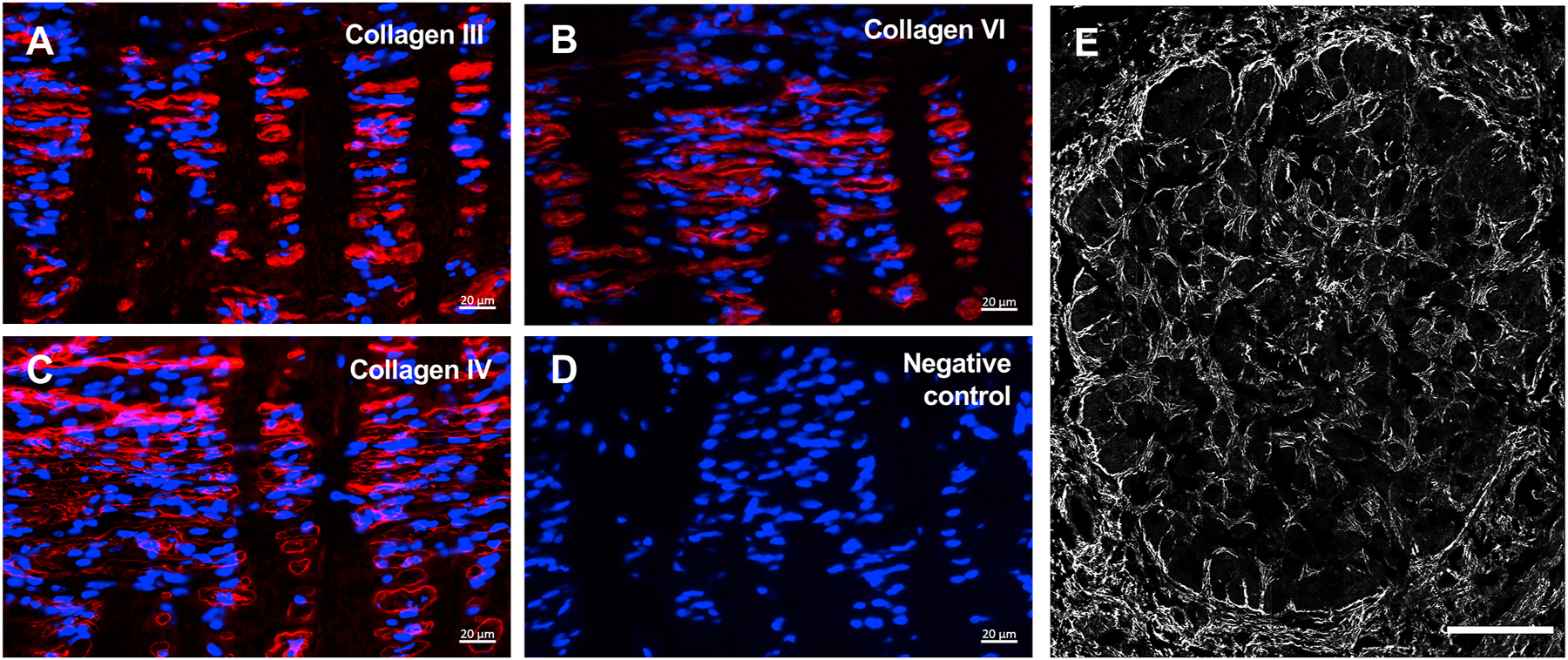
Representative photomicrographs of the lamina cribrosa (LC) region of adult feline optic nerve head (ONH) in longitudinal sections immunolabeled (red) for collagen types III (A), VI (B) and IV (C) and isotype matched immunoglobulin negative control (D) (nuclei counterstained blue with DAPI). (E) Transverse section of the LC region in the ONH immunolabeled for elastin. Scale bars = 20μm (A-D), 200μm (E).
3.3. Distinct resident glial cell populations in sub-regions of the feline ONH
For examination of its cellular components, the feline ONH was divided into three sub-regions (prelaminar, PL; lamina cribrosa, LC; retrolaminar, RL) (Fig. 4). To elucidate the nature of the resident ONH glial cell population and composition in these different sub-regions of the ONH, single and multiplexed immunolabeling was performed with a range of antibodies in either longitudinal or transverse sections of feline ONHs as appropriate. For each of four ONH samples, 3–4 randomly sampled sections were evaluated as technical replicates.
The Pre-laminar (PL) region is characterized by its relative lack of connective tissue, in contrast to the LC region. In this region, unmyelinated RGC axons (NF+ / MBP−) from the RNFL of the inner retina make a turn (Fig. 4E) and pass between well-organized glial columns. The glial columns in the PL region are composed of ONH astrocytes immunopositive for GFAP (a cytoskeletal intermediate filament protein) and SOX9 (an astrocyte specific nuclear transcription factor) (Fig.5A). IBA-1+ myeloid cells are identifiable but are relatively sparse in the PL region. The majority of IBA-1+ cells appear to have an elongated/rod morphology, and are oriented along the course of axons (Fig. 5A).
Fig. 5.

ONH glial cell populations differ between ONH sub-regions. (A) Representative photomicrographs of pre-laminar (PL), lamina cribrosa (LC) and retro-laminar (RL) regions on longitudinal tissue sections of feline optic nerve head immunolabeled for ONH glial cell markers. ONH astrocytes immunolabeled with GFAP (green) and SOX9 (magenta) and microglia with IBA-1 (red), with DAPI counterstained (blue). Differential interference contrast (DIC) signal highlights horizontally aligned connective tissue beams in the LC region. (B) Transverse sections in the LC region immunolabeled for GFAP (green) and neurofilament (NF; magenta) reveal astrocyte processes forming a mesh-like web structure, ramifying within the axon bundles and surrounding RGC axons. (C) Oligodendrocyte lineage cells immunolabeled with OLIG2 (green) and APC (red) were identified only in the RL region. Scale bars = 50μm (A, C), 20μm (B).
Within the Lamina cribrosa (LC) region, the majority of cell nuclei appear elongated and oriented parallel to the LC beams and perpendicular to the longitudinal axis of the optic nerve (Fig.5A). Immunofluorescent labeling of transverse sections of the LC region showed that GFAP+ ONH astrocyte processes extend within the pores of the collagenous LC beams, to form a parallel, mesh-like structure oriented in the same plane as LC beams, through which RGC axons pass. An intimate association between astrocyte processes and RGC axons is apparent (Fig. 5B). IBA-1+ myeloid cells are more numerous within the LC region than in the PL region, and exhibit a horizontally elongated morphology, parallel to the LC beams (Fig. 5A).
The Retro-laminar (RL) region represents the area immediately posterior to the collagenous LC, at which myelination commences in the feline ONH, similar to human and primate ONHs (Fig. 4). In the RL region, mature oligodendrocytes (APC+) and immature oligodendrocytes (OLIG2+APC−) which include oligodendrocyte precursor cells (OPCs), are associated with axons, contributing to the formation of the myelin sheath in this region. (Fig. 5C). Astrocytes in the RL region are oriented parallel to the nerve bundles with processes extending into the axon bundles (Fig. 4). Relative to the PL and LC regions, IBA-1+ microglia/macrophages exhibit more ramified morphology in this region (Fig. 4).
To further examine relative ONH glial cell composition within this sub-regional context, the overall density of ONH cells, as well as the densities of the major glial cell-types identified above were quantified. Our analyses revealed that the densities of glial cells and their relative contribution to the total number of DAPI-stained cells differed significantly between ONH sub-regions (Fig. 6A–C). Astrocytes are the predominant cell-type in feline ONHs, consistent with qualitative reports in human ONHs (Triviño et al., 1996; Ye and Hernandez, 1995). Astrocytes constituted 59% of the total ONH cell population in the feline ONH, with the highest mean proportion (87%) identified in the PL region (Fig. 6D). IBA-1+ myeloid cells (presumed microglia) comprised 7% of the overall ONH cell population, with the lowest density and proportion of overall cell number observed in the PL region (Fig. 6D). Oligodendrocyte lineage cells (identified by immunolabeling for APC [CC-1] and/or OLIG2) make up 40% of the total number of ONH cells in the RL region, the only ONH sub-region in which they were identified, and the location at which myelination of RGC axons commences in the cat (Fig. 6D).
Fig. 6.
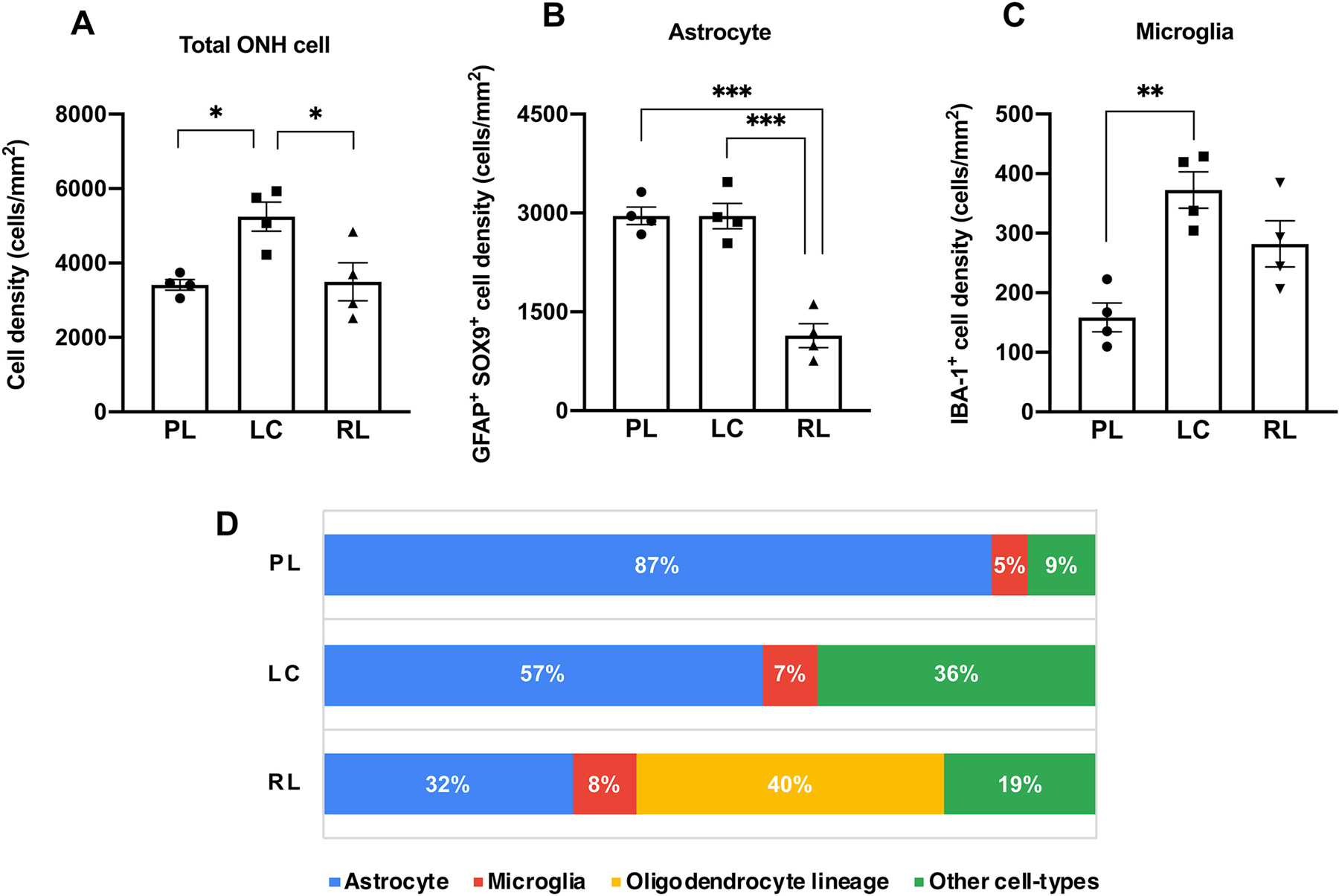
Glial cell composition is heterogeneous within and between ONH sub-regions. Mean densities of total optic nerve head (ONH) cells (A), GFAP+ SOX9+ astrocytes (B) and IBA-1+ microglia (C) in each ONH sub-region. For each cell-type, mean densities were compared between sub-regions (PL; pre-laminar, LC; lamina cribrosa, RL: retro-laminar) by ANOVA followed by Tukey’s multiple comparison correction. Significant differences in densities of total ONH cell, astrocytes and microglia were identified between sub-regions of feline ONHs. (D) A graph showing the relative glial cell proportion in each sub-region of the ONH. Astrocytes are the predominant cell-type in the PL and LC regions, while oligodendrocyte-lineage cells (APC [CC-1]+ and/or OLIG2+) constitute 40% of ONH cells in the RL region. Quantitative data acquired from 4 normal adult cats (with data averaged from 3 ONH sections for each subject examined). Error bars represent standard error of mean. *P <0.05; **P <0.01; *** P <0.005.
3.4. Ultrastructure of the feline ONH
To characterize the ultrastructure of feline ONH, transmission electron microscopy (TEM) was performed, further illustrating that feline ONH closely resembles human and primate ONH (Fig. 7A). In the PL region, fibrous astrocytes form glial columns between non-myelinated axon bundles and parallel the course of the nerve fibers (Fig. 7B), as previously described in human ONH (Anderson, 1969). In this region, fibrous astrocyte processes directly contact RGC axons as well as blood vessels (Fig. 7C). In the LC region, collagenous LC beams are present (Fig. 7D). It is noteworthy that in the sections examined, capillaries were observed within some, but not all, sections through LC collagenous beams. These capillaries, were identifiable as lumens surrounded by endothelial cells and pericytes, and occasionally filled with erythrocytes. (Fig. 7E). In the LC region, astrocytes are localized between and surrounding the connective tissue beams, with their processes extending to, and ramifying within the axon bundles, investing axons that run through the LC pores (Fig. 7E). Consistent with positive MBP and NF-H immunolabeling (shown in Fig. 4B), TEM confirmed that non-myelinated bundles of RGC axons become myelinated in the RL region immediately posterior to the LC (Fig. 7F, G).
Fig. 7.
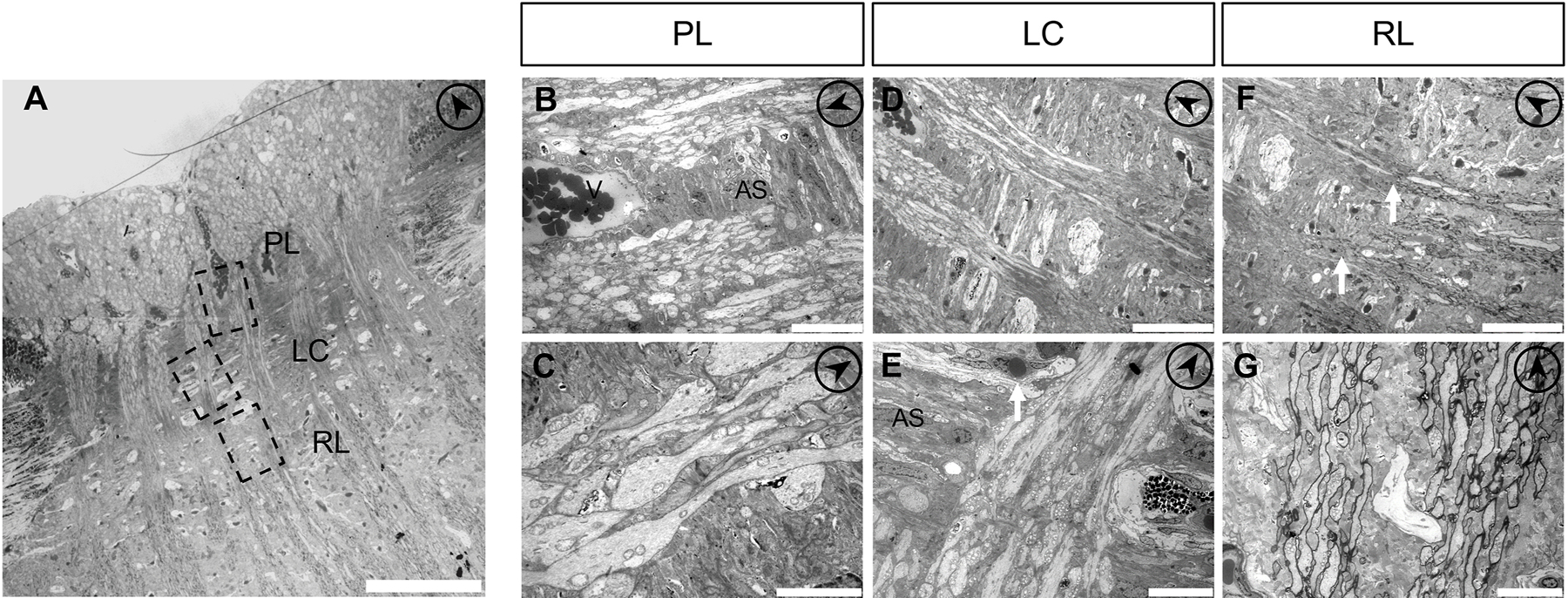
Ultrastructure of the feline optic nerve head. (A) Transmission electron microscopy (TEM) of longitudinal sections of the feline optic nerve head (ONH). (B) In the pre-laminar region (PL) of the ONH, fibrous astrocytes (AS) form glial columns between which retinal ganglion cell axons pass, and directly interact with blood vessels (V). (C) Astrocyte processes extend within axon bundles. (D, E) In the lamina cribrosa region (LC), there are the collagenous beams where capillaries reside (arrow). Numerous mitochondria are observed within RGC axons. Collagenous beams are oriented perpendicular to the RGC axons. Astrocytes (AS) are mostly located between the multi-layered collagenous beams (E). (F, G) In the retro-laminar region (RL). Myelination commences in the RL region (arrows). To aid with orientation, the arrows within circles (upper right on each panel) point anteriorly, towards the anterior surface of the ONH. Scale bars = 100μm (A), 20μm (B), 5μm (C), 50μm (D, F), 10μm (E, G).
3.5. Identification and localization of lamina cribrosa cells in feline ONH
Cells we identified as LC cells, also known as lamina cribrocytes, have only been identified, to our knowledge, in situ in the human ONH (Tovar-Vidales et al., 2016) and are considered as a distinct cell-type among the resident cell population of the ONH. Human LC cells are characterized by expression of α-smooth muscle actin (α-SMA), and a lack of expression of GFAP has been used to distinguish LC cells (GFAP negative) from astrocytes (GFAP positive) (Wallace and O’Brien, 2016; Lopez et al., 2020). To identify LC cells in feline ONH tissue in situ, multiplexed immunofluorescence labeling was carried out with carefully selected markers for LC cells and the other resident ONH cell-types. We identified α-SMA+ GFAP− LC cells in the feline ONH LC region (Fig. 8A, B). Since this α-SMA+ GFAP− cell population in the ONH could also represent pericytes for capillaries or vascular smooth muscle cells (VSMCs) of larger vessels (Bergers and Song, 2005), the association between α-SMA+ LC cells and vascular endothelial cells (labeled with the endothelial marker; von Willebrand Factor [vWF]) was further examined. This revealed that α-SMA+ LC cells were not co-labeled for vWF and did not exhibit close associations with vWF+ endothelial cells (Fig. 8A), indicating that LC cells represent a distinct cell-type from endothelial cells and pericytes/VSMCs. Additionally, LC cells did not express microglia/macrophage marker IBA-1 (Fig. 8B). ONH astrocytes were about 10-fold more numerous than LC cells in the feline LC region (Fig. 8C). Furthermore, on TEM, we observed star-shaped cells with extended processes within the collagenous beams of the feline LC (Fig. 8D), with similar ultrastructural characteristics to LC cells reported previously in humans (Hernandez, 2000). Together, these series of experiments clearly identified a distinct population of LC cells in the feline ONH.
Fig. 8.

A distinct population of lamina cribrosa cells is identifiable in the feline optic nerve head (ONH). (A) Representative photomicrographs of immunolabeled longitudinal sections of normal adult feline ONH in which nuclei are counterstained with DAPI (blue). Lamina cribrosa (LC) cells are positively labeled by α-smooth muscle actin (α-SMA; red) but not by the astrocyte marker GFAP (green). Vascular endothelial cells were immunolabeled by von Willebrand factor (vWF; white). The LC cells do not appear to be in direct contact with vWF+ capillaries in the LC region (arrows), while α-SMA positive pericytes / vascular smooth muscle cells are localized adjacent to vWF+ endothelial cells (arrowheads). (B) Transverse sections of the feline LC region show that α-SMA+ LC cells (arrows) and IBA-1+ microglia/macrophages (white) are located in the GFAP (green) negative area and those markers did not show colocalization. (C) The numbers of ONH astrocytes and of LC cells in the LC region of ONH tissue sections (n=3 technical replicates) from adult cats (n=3) were manually quantified, averaged and compared. ONH astrocytes were significantly more numerous compared to LC cells (P < 0.001; unpaired t-test). (D) Transmission electron micrograph showing the location of a LC cell (white arrow) within a LC collagenous beams (CB). These LC cells appear to extend thin processes (white arrowheads) into the cores of the collagenous beams. In contrast, astrocytes (labeled AS) populate between or surrounding the multi-layered collagenous beams of the LC. Scale bar = 50 μm (A, B), 5 μm (D).
Discussion
The current study demonstrates that feline ONH closely resembles that of humans in relation to microstructure, ECM composition, resident cell-types and ultrastructure.
The feline ONH possesses a robust collagenous LC that consists of laminar stacks of connective tissue beams spanning across the scleral canal. Although how the collagenous LC contributes to pathology of optic neuropathies has yet to be fully understood, a large body of literature has shown that the collagenous LC, together with the peripapillary sclera, plays a critical role in determining ONH biomechanics. The connective tissue elements of the ONH likely function together as a framework that provides the majority of structural support to the ONH, countering IOP at all levels (Yang et al., 2017). Furthermore, deformation, displacement and remodeling of the LC have been consistently shown in glaucoma in human patients, and in non-human primates with experimental glaucoma, but not in the other forms of optic neuropathies (Burgoyne, 2015). Thus, these changes of the LC are considered hallmarks of the pathology of glaucomatous optic neuropathy. Besides primates and feline, a collagenous LC has been reported in a wide variety of species including dogs (Brooks et al., 1989), pigs (Brooks et al., 1998), sheep (Brazile et al., 2018) and horses (Brooks et al., 2000). It is noteworthy that the feline LC structure exhibits a sieve-like collagenous structure that more closely resembles that of humans and non-human primates than the radially oriented collagenous LC beams previously reported in tree shrews (Albon et al., 2007) and guinea pigs (Ostrin and Wildsoet, 2016), species in which the pattern of biomechanical stress and strain are likely to be different from the human ONH. The presence of a robust, sieve-like, collagenous LC in cats, more closely resembling the LC of humans, may be required to recapitulate complex pathological mechanisms, especially biomechanical aspects of glaucomatous optic neuropathy, and could have a significant impact on modeling the disease processes that occur in humans. Further studies are indicated to characterize the biomechanical properties and relative sensitivity to IOP of normal and glaucomatous feline ONHs.
The current study shows that the connective tissue of the LC in cats is composed of collagen types I, III, IV and VI as well as elastin, which is consistent with previous immunohistochemical studies in human and primate ONHs (Hernandez et al., 1994; Morrison et al., 1989a, 1989b; Rehnberg et al., 1987; Wheatley et al., 1995). Both in humans and cats, collagen types I, III and VI are present in the core of laminar beams, while expression of collagen IV was identified in the basement membrane of vessels and at the interface between astrocytes and LC connective tissues. Elastin is expressed in feline LC beams, and is considered to impart strength and resilience to the collagenous LC (Hernandez et al., 1986; Quigley et al., 1991b). Alteration of expression and distribution of these collagens and elastin in the LC region has been shown to occur during the extensive ONH remodeling that characterize human glaucoma (Fukuchi et al., 1992; Hernandez et al., 1994; Morrison et al., 1990; Pena et al., 1998; Quigley et al., 1991a). It has been reported that ECM expression in the human LC is also altered by aging (Albon et al., 2000, 1995; Hernandez et al., 1989). As age is a major risk factor for glaucoma (Tham et al., 2014), further experiments to evaluate the effects of age as well as disease status on these feline ONH ECM components will be critical to our efforts to better understand the pathophysiology of glaucoma in this model.
The majority of the resident cell types identified in the feline ONH are glial cells: including astrocytes, microglia and oligodendrocyte-lineage cells (oligodendrocytes and oligodendrocyte precursor cells [OPC]). Glia play key, pleiotropic roles in homeostasis in the central nervous system and have a profound impact in modulating disease processes (Allen and Lyons, 2018; Li and Barres, 2018). As in humans, our analyses identified astrocytes as the predominant cell-type in the PL and LC regions, in which RGC axons are un-myelinated, whereas oligodendrocyte-lineage cells are the main cell-type in the RL region ONH where RGC axons become myelinated. Consistent with previous studies in human ONH (Wallace and O’Brien, 2016; Lopez et al., 2020), the glia of the feline ONH exhibit distinct resident cell-type composition and well-organized glioarchitecture in each ONH sub-region. Ye and Hernandez (1995) proposed that cellular and functional heterogeneities of astrocytes between the PL, LC and RL regions of human ONH, in turn provides region-specific cellular support to maintain RGC health and synthesize ECM molecules. Intriguingly, recent evidence showed that in the myelin transition zone in mice, corresponding to the RL region in humans and cats, astrocytes appear to have phagocytic activity and contribute to transmitophagy in physiological and pathological conditions (Nguyen et al., 2011), further supporting functional diversity of astrocytes between ONH sub-regions.
As in the human ONH, myelination commences in the RL region of the feline ONH, and oligodendrocyte lineage cells correspondingly dominate the glial population. The myelin sheath allows electrical impulses to transmit efficiently along RGC axons by saltatory conduction, and also provides metabolic support to RGC axons. Studies in experimental monkey glaucoma models suggest that expression of myelin-related proteins is decreased, particularly in the RL region, at early stages of glaucoma prior to significant axon degeneration in the distal optic nerve (Yang et al., 2017), implicating abnormalities in myelin biology in the RL region in glaucoma.
A relatively small subset of ONH cells identified in the normal cats in the current study are microglia and/or macrophages. These myeloid cells play important roles in modulating innate immune responses. Microglial and macrophage activation have been implicated in glaucoma in humans (Neufeld, 1999; Yuan and Neufeld, 2001) as well as animal models (Bosco et al., 2015, 2011; Williams et al., 2016). In a feline model of inherited glaucoma, we have recently identified that ONH microglial activation occurs at early stages of glaucoma even prior to histologically detectable axon degeneration (Oikawa et al., 2020), highlighting an important link between neuroinflammation and pathogenesis and/or progression of glaucoma.
Together, our results show that sub-region-specific glial cell populations and their composition in the feline ONH are similar to humans. Leveraging the results of the current study, ongoing studies in our lab seek to determine sub-region- and cell-type-specific molecular and functional responses of ONH glial cells in glaucomatous cats. To the authors’ knowledge, this is the first report documenting LC cells in situ in the ONH of an animal species used as a model for glaucoma research. LC cells were initially isolated and cultured from postmortem human ONH tissue homogenate (Hernandez et al., 1988), and identified in human ONH tissues in situ (Tovar-Vidales et al., 2016). In this study, feline LC cells were identified using the same markers reported for human LC cells (positive expression of α-SMA and negative expression of GFAP). Electron microscopy also demonstrated that the feline LC cell is qualitatively similar in appearance to the human LC cell which, as reported by Hernandez (2000) is star-shaped, resides within collagenous beams and extends thin processes in the core of the LC beams. Although glial cells predominate in the ONH, numerous in vitro studies on cultured human LC cells have suggested that LC cells may play an important role in glaucoma by regulating ECM production (Kirwan et al., 2005a; Kirwan et al., 2005b; Paula et al., 2016). Additionally, LC cells isolated from human donors with glaucoma also exhibit higher expression levels of pro-fibrotic genes, compared to LC cells from non-glaucomatous donors (Kirwan et al., 2009). Together, these studies implicate LC cells in ECM remodeling in glaucomatous optic neuropathy. However, studies of LC biology in glaucoma in vivo and in situ are lacking, due to a lack of appropriate animal models. Importantly, our findings identify a promising animal model to study the role of LC cells in situ under both physiological and pathological conditions.
In addition to glial cells, which constitute a large proportion of ONH cells, there is a further subset of cells in the feline ONH that do not express glial markers, in addition to the LC cells described above. These cells include fibroblasts as well as cells associated with vessels and capillaries. In particular, the vasculature of the ONH plays a critical role in maintaining a healthy ONH microenvironment. The feline ONH lacks a central retinal artery and vein (Wong and Macri, 1964). However, as in humans, ONH blood supply mainly relies on a complex vascular network arising from the short posterior ciliary arteries and their derivative choroidal arteries and pial vessels (Risco and Nopanitaya, 1980; Wong and Macri, 1964). Immunolabeling and electron microscopy results demonstrated capillaries within the core of the collagenous LC beams in cats, as in the human ONH (Levitzky and Henkind, 1969). Microcapillaries in the core of LC beams are considered to provide the nutritional support to resident ONH cells as well as RGC axons. Biomechanical stress and strain related to IOP could compromise vascular function of LC microcapillaries and overall blood supply to the ONH, with detrimental effects on RGC axonal health. However, the nature of interactions between resident ONH cells, including LC cells, and capillaries within the core of LC beams have not been fully elucidated and warrant further investigation.
In conclusion, the current study establishes many important similarities between the ONH of cats and humans. Our findings provide a premise for ongoing and future studies in cats aimed at understanding the complex roles of ECM of the LC, resident glia, LC cells and microvasculature in the cellular and molecular pathobiology of the ONH in glaucoma, leveraging our unique glaucoma model.
Highlights.
Feline ONH has a robust collagenous LC with similar microstructure and ECM composition to that of humans.
A distinct population of LC cells is present in the feline ONH.
Glia, comprised of astrocytes, microglia and oligodendrocytes, collectively represent the major cell-type of the feline ONH.
Distinct sub-regions of the feline ONH differ substantially in their composition of cell types.
The feline eye provides a translationally relevant model for glaucoma research.
Acknowledgment
The authors would like to thank Ben August for assistance with EM tissue processing and sectioning, and Satoshi Kinoshita for preparation of cryosections.
Funding
This study was supported by funding from NIH R01 EY027396 (GJM) and P30 EY016665; a BrightFocus Foundation National Glaucoma Research Award (GJM); Retina Research Foundation Walter H. Helmerich Professorship (KWE); a Japan Student Services Organization Scholarship (KO); a Comparative Biomedical Sciences Dissertation Completion Fellowship (KO), and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison.
Abbreviations
- APC
Adenomatous polyposis coli
- BSA
Bovine serum albumin
- DIC
Differential interference contrast
- ECM
Extracellular matrix
- FCG
Feline congenital glaucoma
- GFAP
Glial fibrillary acidic protein
- H&E
Hematoxylin and eosin
- IBA-1
Ionized calcium-binding adapter molecule-1
- IOP
Intraocular pressure
- LC
Lamina cribrosa
- MBP
Myelin basic protein
- NF
Neurofilament
- OLIG2
Oligodendrocyte transcription factor 2
- ONH
Optic nerve head
- PFA
Paraformaldehyde
- PL
Pre-laminar
- RGC
Retinal ganglion cell
- RL
Retro-laminar
- SHG
Second harmonic generation
- SOX9
SRY-Box transcription factor 9
- TEM
Transmission electron microscopy
- vWF
von Willebrand Factor
- α-SMA
Alpha-smooth muscle actin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting interest statement
The authors declare that they have no competing financial interests.
References
- Adelman S, Shinsako D, Kiland JA, Yaccarino V, Ellinwood NM, Ben-Shlomo G, McLellan GJ, 2018. The post-natal development of intraocular pressure in normal domestic cats (Felis catus) and in feline congenital glaucoma. Exp. Eye Res 166, 70–73. 10.1016/j.exer.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, Farrant S, Akhtar S, Young R, Boulton ME, Smith G, Taylor M, Guggenheim J, Morgan JE, 2007. Connective tissue structure of the tree shrew optic nerve and associated ageing changes. Invest. Ophthalmol. Vis. Sci 48, 2134–2144. 10.1167/iovs.06-0084 [DOI] [PubMed] [Google Scholar]
- Albon J, Karwatowski WS, Avery N, Easty DL, Duance VC, 1995. Changes in the collagenous matrix of the aging human lamina cribrosa. Br. J. Ophthalmol 79, 368–375. 10.1136/bjo.79.4.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, Karwatowski WS, Easty DL, Sims TJ, Duance VC, 2000. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br. J. Ophthalmol 84, 311–317. 10.1136/bjo.84.3.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy A-L, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF, 2009. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet 84, 664–671. 10.1016/j.ajhg.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Lyons DA, 2018. Glia as architects of central nervous system formation and function. Science 362, 181–185. 10.1126/science.aat0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR, 1969. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch. Ophthalmol. Chic. Ill 1960 82, 800–814. 10.1001/archopht.1969.00990020792015 [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Kang MH, Yu P, Chan G, Morgan WH, Cringle SJ, Yu D-Y, 2014. Comparative quantitative study of astrocytes and capillary distribution in optic nerve laminar regions. Exp. Eye Res 121, 11–22. 10.1016/j.exer.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Johnstone V, Cringle SJ, Yu D-Y, 2009. Heterogeneous distribution of axonal cytoskeleton proteins in the human optic nerve. Invest. Ophthalmol. Vis. Sci 50, 2824–2838. 10.1167/iovs.08-3206 [DOI] [PubMed] [Google Scholar]
- Barron MJ, Griffiths P, Turnbull DM, Bates D, Nichols P, 2004. The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br. J. Ophthalmol 88, 286–290. 10.1136/bjo.2003.027664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, 2005. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncol 7, 452–464. 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, Vetter ML, 2015. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech 8, 443–455. 10.1242/dmm.018788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Steele MR, Vetter ML, 2011. Early microglia activation in a mouse model of chronic glaucoma. J. Comp. Neurol 519, 599–620. 10.1002/cne.22516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazile BL, Hua Y, Jan N-J, Wallace J, Gogola A, Sigal IA, 2018. Thin Lamina Cribrosa Beams Have Different Collagen Microstructure Than Thick Beams. Invest. Ophthalmol. Vis. Sci 59, 4653–4661. 10.1167/iovs.18-24763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM, 2002. The distribution of mitochondrial activity in relation to optic nerve structure. Arch. Ophthalmol. Chic. Ill 1960 120, 791–796. 10.1001/archopht.120.6.791 [DOI] [PubMed] [Google Scholar]
- Brooks DE, Arellano E, Kubilis PS, Komaromy AM, 1998. Histomorphometry of the porcine scleral lamina cribrosa surface. Vet. Ophthalmol 1, 129–135. 10.1046/j.1463-5224.1998.00029.x [DOI] [PubMed] [Google Scholar]
- Brooks DE, Komaromy AM, Garcia-Fernandez MC, Cutler TJ, Samuelson DA, Kallberg ME, 2000. Immunohistochemistry of the extracellular matrix of the normal equine lamina cribrosa. Vet. Ophthalmol 3, 127–132. 10.1046/j.1463-5224.2000.00127.x [DOI] [PubMed] [Google Scholar]
- Brooks DE, Samuelson DA, Gelatt KN, Smith PJ, 1989. Morphologic changes in the lamina cribrosa of beagles with primary open-angle glaucoma. Am. J. Vet. Res 50, 936–941. [PubMed] [Google Scholar]
- Burgoyne C, 2015. The morphological difference between glaucoma and other optic neuropathies. J. Neuro-Ophthalmol. Off. J. North Am. Neuro-Ophthalmol. Soc 35 Suppl 1, S8–S21. 10.1097/WNO.0000000000000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Weber AJ, 2001. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest. Ophthalmol. Vis. Sci 42, 966–974. [PubMed] [Google Scholar]
- Dichtl A, Jonas JB, Naumann GO, 1996. Course of the optic nerve fibers through the lamina cibrosa in human eyes. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 234, 581–585. 10.1007/BF00448803 [DOI] [PubMed] [Google Scholar]
- Downs JC, Girkin CA, 2017. Lamina cribrosa in glaucoma. Curr. Opin. Ophthalmol 28, 113–119. 10.1097/ICU.0000000000000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington AR, Inman CB, Steart PV, Weller RO, 1990. The structure of the lamina cribrosa of the human eye: an immunocytochemical and electron microscopical study. Eye Lond. Engl 4 ( Pt 1), 42–57. 10.1038/eye.1990.5 [DOI] [PubMed] [Google Scholar]
- Franchi M, Raspanti M, Dell’Orbo C, Quaranta M, De Pasquale V, Ottani V, Ruggeri A, 2008. Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect. Tissue Res 49, 85–91. 10.1080/03008200801913635 [DOI] [PubMed] [Google Scholar]
- Fukuchi T, Sawaguchi S, Hara H, Shirakashi M, Iwata K, 1992. Extracellular matrix changes of the optic nerve lamina cribrosa in monkey eyes with experimentally chronic glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 230, 421–427. 10.1007/BF00175926 [DOI] [PubMed] [Google Scholar]
- Goldbaum MH, Jeng SY, Logemann R, Weinreb RN, 1989. The extracellular matrix of the human optic nerve. Arch. Ophthalmol. Chic. Ill 1960 107, 1225–1231. 10.1001/archopht.1989.01070020291041 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, 2000. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog. Retin. Eye Res 19, 297–321. 10.1016/s1350-9462(99)00017-8 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Igoe F, Neufeld AH, 1988. Cell culture of the human lamina cribrosa. Invest. Ophthalmol. Vis. Sci 29, 78–89. [PubMed] [Google Scholar]
- Hernandez MR, Igoe F, Neufeld AH, 1986. Extracellular matrix of the human optic nerve head. Am. J. Ophthalmol 102, 139–148. 10.1016/0002-9394(86)90134-0 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Luo XX, Andrzejewska W, Neufeld AH, 1989. Age-related changes in the extracellular matrix of the human optic nerve head. Am. J. Ophthalmol 107, 476–484. 10.1016/0002-9394(89)90491-1 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Ye H, Roy S, 1994. Collagen type IV gene expression in human optic nerve heads with primary open angle glaucoma. Exp. Eye Res 59, 41–51. 10.1006/exer.1994.1079 [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SWM, 2007. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol 179, 1523–1537. 10.1083/jcb.200706181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan N-J, Gomez C, Moed S, Voorhees AP, Schuman JS, Bilonick RA, Sigal IA, 2017. Microstructural Crimp of the Lamina Cribrosa and Peripapillary Sclera Collagen Fibers. Invest. Ophthalmol. Vis. Sci 58, 3378–3388. 10.1167/iovs.17-21811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Mardin CY, Schlötzer-Schrehardt U, Naumann GO, 1991. Morphometry of the human lamina cribrosa surface. Invest. Ophthalmol. Vis. Sci 32, 401–405. [PubMed] [Google Scholar]
- Kang MH, Law-Davis S, Balaratnasingam C, Yu D-Y, 2014. Sectoral variations in the distribution of axonal cytoskeleton proteins in the human optic nerve head. Exp. Eye Res 128, 141–150. 10.1016/j.exer.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Kang MH, Suo M, Balaratnasingam C, Yu PK, Morgan WH, Yu D-Y, 2018. Microvascular Density Is Associated With Retinal Ganglion Cell Axonal Volume in the Laminar Compartments of the Human Optic Nerve Head. Invest. Ophthalmol. Vis. Sci 59, 1562–1570. 10.1167/iovs.17-23183 [DOI] [PubMed] [Google Scholar]
- Kang MH, Yu D-Y, 2015. Distribution pattern of axonal cytoskeleton proteins in the human optic nerve head. Neural Regen. Res 10, 1198–1200. 10.4103/1673-5374.162691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan Ruaidhrí P., Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ, 2005a. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol. Vis 11, 798–810. [PubMed] [Google Scholar]
- Kirwan Ruaidhrí P, Leonard MO, Murphy M, Clark AF, O’Brien CJ, 2005b. Transforming growth factor-β-regulated gene transcription and protein expression in human GFAP-negative lamina cribrosa cells. Glia 52, 309–324. 10.1002/glia.20247 [DOI] [PubMed] [Google Scholar]
- Kirwan RP, Wordinger RJ, Clark AF, O’Brien CJ, 2009. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol. Vis 15, 76–88. [PMC free article] [PubMed] [Google Scholar]
- Kuehn MH, Lipsett KA, Menotti-Raymond M, Whitmore SS, Scheetz TE, David VA, O’Brien SJ, Zhao Z, Jens JK, Snella EM, Ellinwood NM, McLellan GJ, 2016. A Mutation in LTBP2 Causes Congenital Glaucoma in Domestic Cats (Felis catus). PLoS ONE 11, e0154412 10.1371/journal.pone.0154412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z, EMGT Group, 2007. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114, 1965–1972. 10.1016/j.ophtha.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Levitzky M, Henkind P, 1969. Angioarchitecture of the optic nerve. II. Lamina cribrosa. Am. J. Ophthalmol 68, 986–996. 10.1016/0002-9394(69)93438-2 [DOI] [PubMed] [Google Scholar]
- Li Q, Barres BA, 2018. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol 18, 225–242. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- Lopez NN, Clark AF, Tovar-Vidales T, 2020. Isolation and characterization of human optic nerve head astrocytes and lamina cribrosa cells. Exp. Eye Res 197, 108103 10.1016/j.exer.2020.108103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CA, Lütjen-Drecoll E, 2002. Morphology of the murine optic nerve. Invest. Ophthalmol. Vis. Sci 43, 2206–2212. [PubMed] [Google Scholar]
- McLellan GJ, Teixeira LBC, 2015. Feline Glaucoma. Vet. Clin. North Am. Small Anim. Pract 45, 1307–1333. 10.1016/j.cvsm.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Minckler DS, McLean IW, Tso MO, 1976. Distribution of axonal and glial elements in the rhesus optic nerve head studied by electron microscopy. Am. J. Ophthalmol 82, 179–187. 10.1016/0002-9394(76)90416-5 [DOI] [PubMed] [Google Scholar]
- Morrison J, Farrell S, Johnson E, Deppmeier L, Moore CG, Grossmann E, 1995. Structure and composition of the rodent lamina cribrosa. Exp. Eye Res 60, 127–135. 10.1016/s0014-4835(95)80002-6 [DOI] [PubMed] [Google Scholar]
- Morrison JC, Dorman-Pease ME, Dunkelberger GR, Quigley HA, 1990. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch. Ophthalmol. Chic. Ill 1960 108, 1020–1024. 10.1001/archopht.1990.01070090122053 [DOI] [PubMed] [Google Scholar]
- Morrison JC, Jerdan JA, Dorman ME, Quigley HA, 1989a. Structural proteins of the neonatal and adult lamina cribrosa. Arch. Ophthalmol. Chic. Ill 1960 107, 1220–1224. 10.1001/archopht.1989.01070020286040 [DOI] [PubMed] [Google Scholar]
- Morrison JC, L’Hernault NL, Jerdan JA, Quigley HA, 1989b. Ultrastructural location of extracellular matrix components in the optic nerve head. Arch. Ophthalmol. Chic. Ill 1960 107, 123–129. 10.1001/archopht.1989.01070010125040 [DOI] [PubMed] [Google Scholar]
- Narooie-Nejad M, Paylakhi SH, Shojaee S, Fazlali Z, Rezaei Kanavi M, Nilforushan N, Yazdani S, Babrzadeh F, Suri F, Ronaghi M, Elahi E, Paisán-Ruiz C, 2009. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum. Mol. Genet 18, 3969–3977. 10.1093/hmg/ddp338 [DOI] [PubMed] [Google Scholar]
- Neufeld AH, 1999. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch. Ophthalmol 117, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Ng A, Stone J, 1982. The optic nerve of the cat: appearance and loss of axons during normal development. Dev. Brain Res 5, 263–271. 10.1016/0165-3806(82)90125-0 [DOI] [PubMed] [Google Scholar]
- Nguyen JV, Soto I, Kim K-Y, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CO, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N, 2011. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. U. S. A 108, 1176–1181. 10.1073/pnas.1013965108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SWM, 2012. Under Pressure: Cellular and Molecular Responses During Glaucoma, a Common Neurodegeneration with Axonopathy. Annu. Rev. Neurosci 35, 153–179. 10.1146/annurev.neuro.051508.135728 [DOI] [PubMed] [Google Scholar]
- Oikawa K, Ver Hoeve JN, Teixeira LBC, Snyder KC, Kiland JA, Ellinwood NM, McLellan GJ, 2020. Sub-region-Specific Optic Nerve Head Glial Activation in Glaucoma. Mol. Neurobiol 57, 2620–2638. 10.1007/s12035-020-01910-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Wildsoet CF, 2016. Optic nerve head and intraocular pressure in the guinea pig eye. Exp. Eye Res 146, 7–16. 10.1016/j.exer.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Abe H, Ushiki T, 2006. The connective tissue and glial framework in the optic nerve head of the normal human eye: light and scanning electron microscopic studies. Arch. Histol. Cytol 69, 341–356. 10.1679/aohc.69.341 [DOI] [PubMed] [Google Scholar]
- Paula JS, O’Brien C, Stamer WD, 2016. Life under pressure: The role of ocular cribriform cells in preventing glaucoma. Exp. Eye Res 151, 150–159. 10.1016/j.exer.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JD, Netland PA, Vidal I, Dorr DA, Rasky A, Hernandez MR, 1998. Elastosis of the lamina cribrosa in glaucomatous optic neuropathy. Exp. Eye Res 67, 517–524. 10.1006/exer.1998.0539 [DOI] [PubMed] [Google Scholar]
- Quigley HA, Brown A, Dorman-Pease ME, 1991a. Alterations in elastin of the optic nerve head in human and experimental glaucoma. Br. J. Ophthalmol 75, 552–557. 10.1136/bjo.75.9.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Dorman-Pease ME, Brown AE, 1991b. Quantitative study of collagen and elastin of the optic nerve head and sclera in human and experimental monkey glaucoma. Curr. Eye Res 10, 877–888. 10.3109/02713689109013884 [DOI] [PubMed] [Google Scholar]
- Quigley HA, Green WR, 1979. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology 86, 1803–1830. 10.1016/s0161-6420(79)35338-6 [DOI] [PubMed] [Google Scholar]
- Radius RL, Bade B, 1982a. Axonal transport interruption and anatomy at the lamina cribrosa. Arch. Ophthalmol. Chic. Ill 1960 100, 1661–1664. 10.1001/archopht.1982.01030040639017 [DOI] [PubMed] [Google Scholar]
- Radius RL, Bade B, 1982b. The anatomy at the lamina cribrosa in the normal cat eye. Arch. Ophthalmol. Chic. Ill 1960 100, 1658–1660. 10.1001/archopht.1982.01030040636016 [DOI] [PubMed] [Google Scholar]
- Radius RL, Bade B, 1981. Pressure-induced optic nerve axonal transport interruption in cat eyes. Arch. Ophthalmol. Chic. Ill 1960 99, 2163–2165. 10.1001/archopht.1981.03930021039011 [DOI] [PubMed] [Google Scholar]
- Radius RL, Gonzales M, 1981. Anatomy of the lamina cribrosa in human eyes. Arch. Ophthalmol 99, 2159–2162. 10.1001/archopht.1981.03930021035010 [DOI] [PubMed] [Google Scholar]
- Rehnberg M, Ammitzböll T, Tengroth B, 1987. Collagen distribution in the lamina cribrosa and the trabecular meshwork of the human eye. Br. J. Ophthalmol 71, 886–892. 10.1136/bjo.71.12.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco JM, Nopanitaya W, 1980. Ocular microcirculation. Scanning electron microscopic study. Invest. Ophthalmol. Vis. Sci 19, 5–12. [PubMed] [Google Scholar]
- Rutz-Mendicino MM, Snella EM, Jens JK, Gandolfi B, Carlson SA, Kuehn MH, McLellan GJ, Ellinwood NM, 2011. Removal of potentially confounding phenotypes from a Siamese-derived feline glaucoma breeding colony. Comp. Med 61, 251–257. [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EM, Teixeira LBC, Flanders DJ, Dubielzig RR, McLellan GJ, 2016. Canine orbital rhabdomyosarcoma: a report of 18 cases. Vet. Ophthalmol 19, 130–137. 10.1111/vop.12270 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD, 1982. Organization of visual pathways in normal and visually deprived cats. Physiol. Rev 62, 738–855. 10.1152/physrev.1982.62.2.738 [DOI] [PubMed] [Google Scholar]
- Shou T, Liu J, Wang W, Zhou Y, Zhao K, 2003. Differential dendritic shrinkage of alpha and beta retinal ganglion cells in cats with chronic glaucoma. Invest. Ophthalmol. Vis. Sci 44, 3005–3010. 10.1167/iovs.02-0620 [DOI] [PubMed] [Google Scholar]
- Siliprandi R, Bucci MG, Canella R, Carmignoto G, 1988. Flash and pattern electroretinograms during and after acute intraocular pressure elevation in cats. Invest. Ophthalmol. Vis. Sci 29, 558–565. [PubMed] [Google Scholar]
- Snyder KC, Oikawa K, Williams J, Kiland JA, Gehrke S, Teixeira LBC, Huang AS, McLellan GJ, 2019. Imaging Distal Aqueous Outflow Pathways in a Spontaneous Model of Congenital Glaucoma. Transl. Vis. Sci. Technol 8, 22 10.1167/tvst.8.5.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thale A, Tillmann B, Rochels R, 1996. SEM studies of the collagen architecture of the human lamina cribrosa: normal and pathological findings. Ophthalmol. J. Int. Ophtalmol. Int. J. Ophthalmol. Z. Augenheilkd 210, 142–147. 10.1159/000310694 [DOI] [PubMed] [Google Scholar]
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y, 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Wordinger RJ, Clark AF, 2016. Identification and localization of lamina cribrosa cells in the human optic nerve head. Exp. Eye Res 147, 94–97. 10.1016/j.exer.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Triviño A, Ramírez JM, Salazar JJ, Ramírez AI, García-Sánchez J, 1996. Immunohistochemical study of human optic nerve head astroglia. Vision Res 36, 2015–2028. 10.1016/0042-6989(95)00317-7 [DOI] [PubMed] [Google Scholar]
- Wallace DM, O’Brien CJ, 2016. The role of lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Exp. Eye Res 142, 102–109. 10.1016/j.exer.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The pathophysiology and treatment of glaucoma: a review. JAMA 311, 1901–1911. 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley HM, Traboulsi EI, Flowers BE, Maumenee IH, Azar D, Pyeritz RE, Whittum-Hudson JA, 1995. Immunohistochemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch. Ophthalmol. Chic. Ill 1960 113, 103–109. 10.1001/archopht.1995.01100010105028 [DOI] [PubMed] [Google Scholar]
- Williams PA, Marsh-Armstrong N, Howell GR, Bosco A, Danias J, Simon J, Di Polo A, Kuehn MH, Przedborski S, Raff M, Trounce I, 2017. Neuroinflammation in glaucoma: A new opportunity. Exp. Eye Res 157, 20–27. 10.1016/j.exer.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SWM, Howell GR, 2016. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener 1–13. 10.1186/s13024-016-0091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VG, Macri FJ, 1964. VASCULATURE OF THE CAT EYE. Arch. Ophthalmol. Chic. Ill 1960 72, 351–358. 10.1001/archopht.1964.00970020351013 [DOI] [PubMed] [Google Scholar]
- Yang H, Reynaud J, Lockwood H, Williams G, Hardin C, Reyes L, Stowell C, Gardiner SK, Burgoyne CF, 2017. The connective tissue phenotype of glaucomatous cupping in the monkey eye - Clinical and research implications. Prog. Retin. Eye Res 59, 1–52. 10.1016/j.preteyeres.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Hernandez MR, 1995. Heterogeneity of astrocytes in human optic nerve head. J. Comp. Neurol 362, 441–452. 10.1002/cne.903620402 [DOI] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH, 2001. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res 64, 523–532. 10.1002/jnr.1104 [DOI] [PubMed] [Google Scholar]


