Extended Data Fig. 6: DNA pair selection and timecourse of INO80 remodeling.
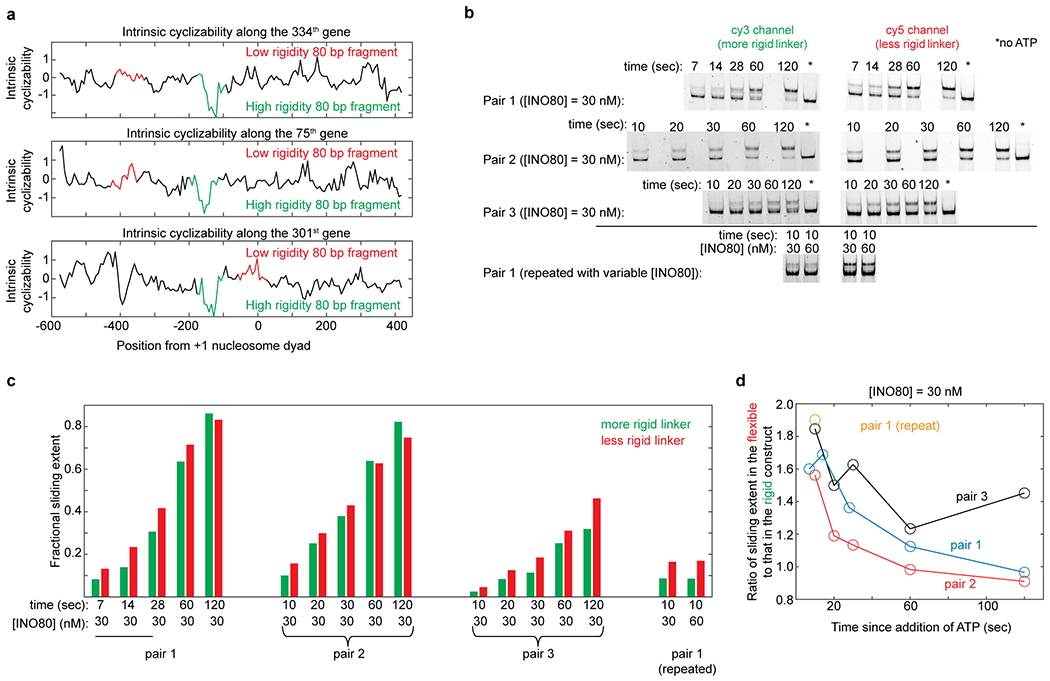
a, Intrinsic cyclizability as a function of position from the +1 nucleosomal dyad, along the 334th, 75th, and 301st genes in the list of 576 genes along which intrinsic cyclizability was measured (Supplementary Note 9). The 80 bp linker regions of both constructs (with rigid and flexible linkers extending from the 601 sequence) in pairs 1, 2, and 3 along which INO80 sliding extent was measured, were selected from the 334th, 75th, and 301st genes respectively. Genes are oriented in the upstream to downstream direction. Red and green denote the selected 80 bp less rigid and more rigid linker regions respectively. See Supplementary Note 11. b, The remodeling reactions shown in Fig. 2f were all performed for 1 minute of remodeling, under various enzyme concentrations. Here we show, instead, remodeling reaction timecourses at saturating [INO80] for all three pairs. Conditions are identical to that used in Fig. 2f, except that saturating (30 nM) INO80 is used and remodeling is permitted to progress for various amounts of time after addition of ATP (see methods). In all cases, the two constructs in a pair are present simultaneously, and distinguished by imaging the gel once for Cy3 and once for Cy5 fluorescence. Thus, although the sliding extent can be very sensitive to sliding time (especially for short sliding times), robust comparisons of sliding extents can be made between the two constructs in a pair. Quantification is done as explained in supplementary note 11. Sliding on the constructs in pair 1 was repeated in a separate experiment in presence of 30 nM enzyme, and also side by side in presence of 60 nM enzyme. Near identical extents of sliding indicate saturation has been reached at 30 nM INO80. c, Ratio of the fractional sliding extent in the construct formed on the more flexible linker to that formed on the more rigid linker, at various timepoints since addition of ATP, and in presence of 30 nM INO80. The dashed line indicates a ratio of 1. The ratio is computed from the data in panel b. The extent of sliding under saturating enzyme conditions is consistently higher for the construct involving more flexible linker (except, as expected, when the sliding extent approaches 100%). Solid lines connect observations that were made from the same initial reaction volume by sampling its fractions at various timepoints.
