Figure 4.
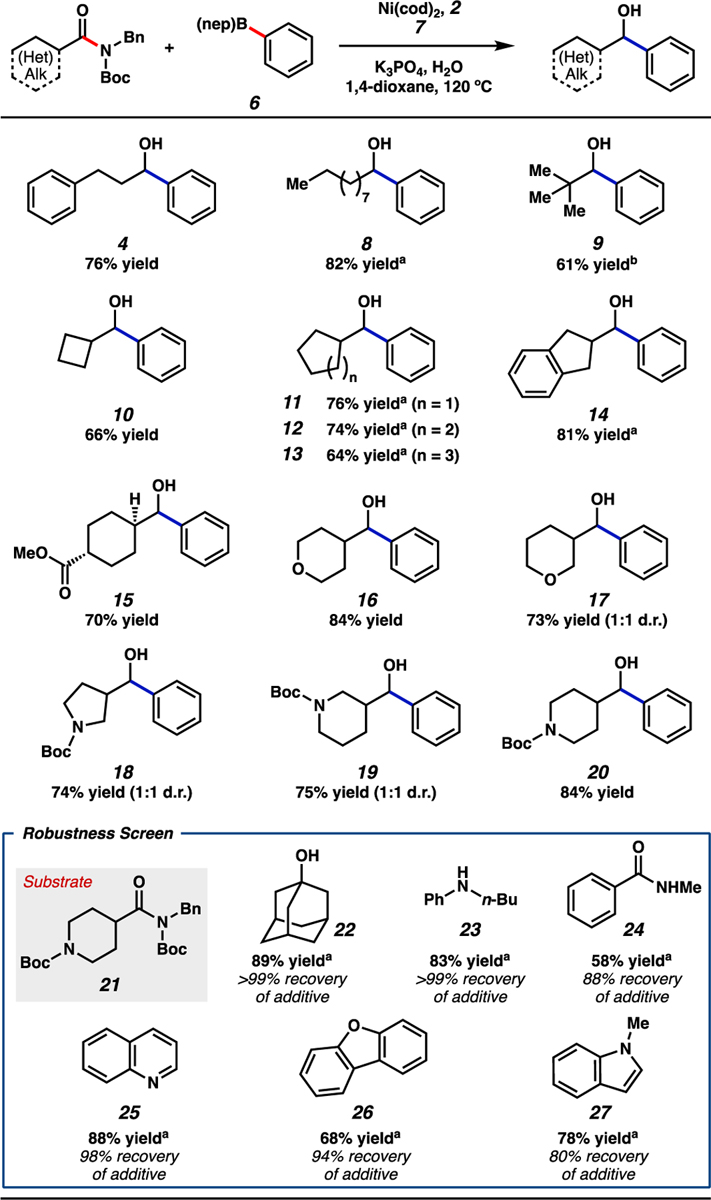
Scope of the reductive arylation of aliphatic amides and boronate 6. Standard conditions unless otherwise noted: amide substrate (0.20 mmol, 1.0 equiv); phenyl boronate 6 (0.80 mmol, 4.0 equiv); 7 (0.50 mmol, 2.5 equiv); K3PO4 (0.80 mmol, 4.0 equiv); H2O (0.40 mmol, 2.0 equiv); Ni(cod)2 (0.020 mmol, 10 mol%); 2 (0.040 mmol, 20 mol%); solvent (1.0 M); 120 °C; 16 h. Unless otherwise noted, yields reflect the average of two isolation experiments. [a] Yield determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an external standard. [b] Reaction ran at 130 °C.
