Fig. 2 |. Structural basis for the microtubule docking and longitudinal periodicity of radial spokes.
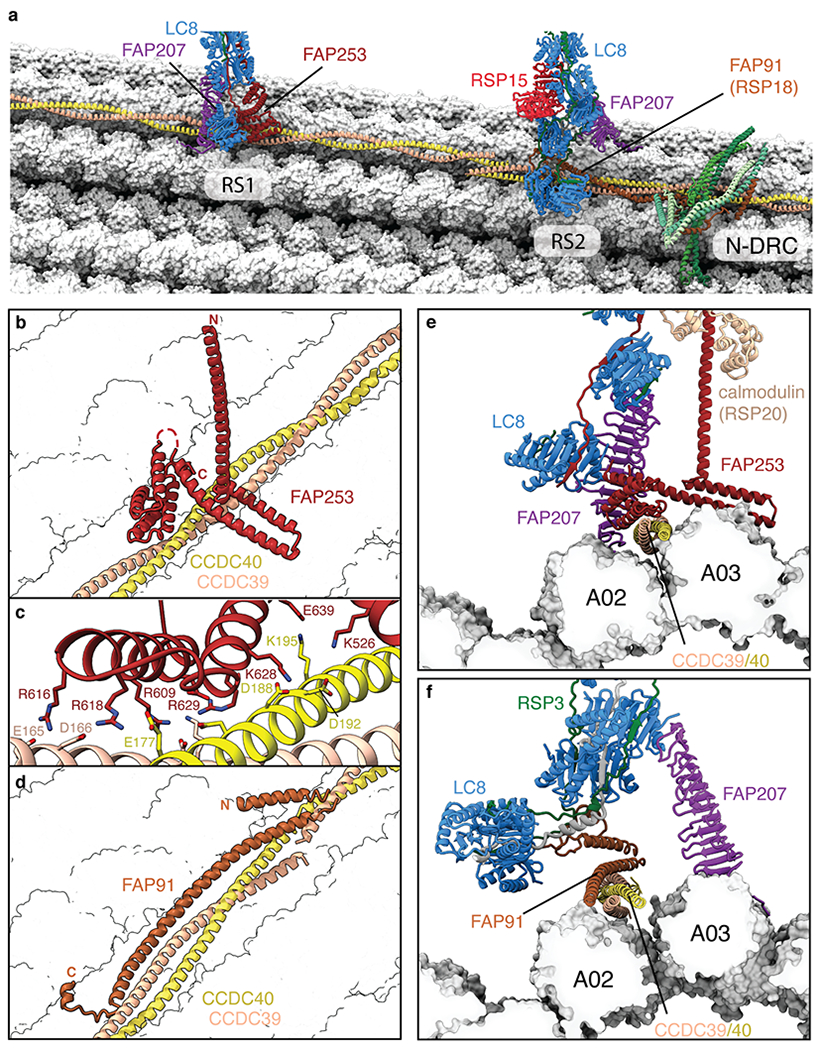
a, Overview showing the bases of radial spokes 1 and 2 (RS1 and RS2) bound to the doublet microtubule. The radial spoke subunits (LC8, FAP91, FAP207, FAP253, and RSP15), the CCDC39/40 molecular ruler, and the subunits of the nexin-dynein regulatory complex (N-DRC) baseplate are shown as cartoons. Tubulin is shown in surface representation. b, The binding site for RS1 is determined by recognition of CCDC39/40 by FAP253. c, Zoom-in view showing that the interface between FAP253 and CCDC39/40 is dominated by a network of salt bridges. d, The binding site for RS2 is determined by FAP91 (RSP18) which forms a triple coiled coil with CCDC39/40. e, Cross-section view of the RS1-doublet microtubule interaction showing recognition of CCDC39/40 by FAP253. FAP207 bridges LC8 with protofilament A02. f, Cross-section view of the RS2-doublet microtubule interaction showing recognition of CCDC39/40 by FAP91. FAP207 bridges LC8 with protofilament A03.
