Fig. 6 |. Molecular basis for the control of IDA motor activity by mechanical signals.
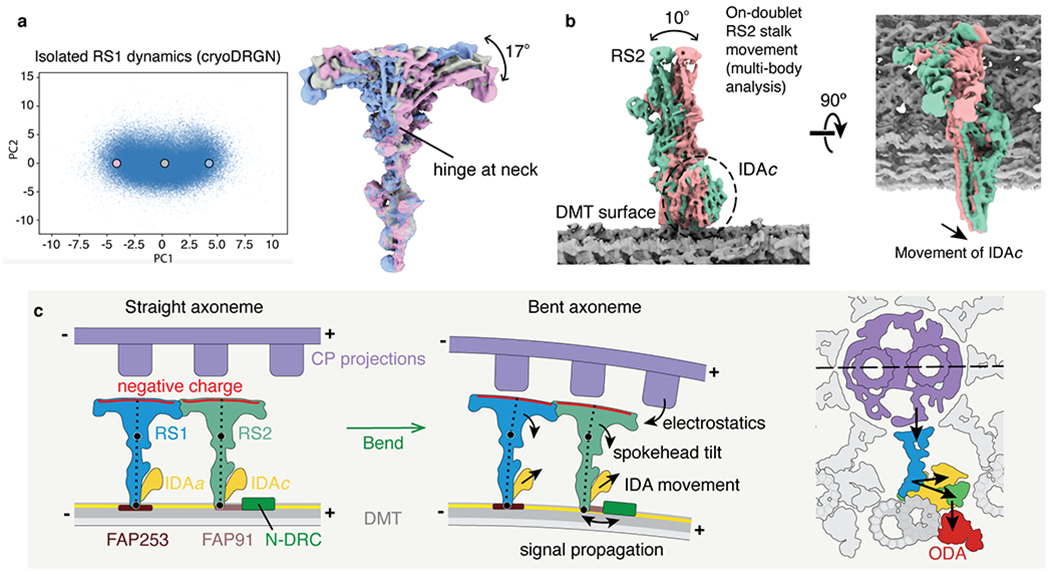
a, Conformational dynamics of isolated radial spoke 1 (RS1) inferred from deep neural networks. Left, Principle component (PC) analysis projection of latent space. Density maps were generated at three points along PC1. Right, Density maps generated from these 3 points were aligned on the stalk and show tilting of the spokehead relative to the stalk. The hinge lies in the neck. b, Multi-body analysis of the movement of the RS2 stalk and IDAc with respect to the doublet microtubule (DMT) surface. The DMT, RS2, and IDAc were separately masked and treated as individual bodies free to move relative to one another. Movement of IDAc corresponds with the movement of the RS2 stalk. c, A model for mechanoregulation of ciliary motility. As the axoneme bends, the projections of the central pair (CP) are brought closer to the spokeheads. An increase in the electrostatic force with decreasing distance causes the radial spoke to tilt at two hinge points (indicated with black circles). As the movement of IDAc is coupled to the movement of RS2, tilting of the radial spoke changes the orientation of the IDA. IDAa may likewise be coupled to the movement of RS1. Signals may propagate from RS2 to N-DRC through FAP91 and the CCDC39/40 coiled coil and from the N-DRC to the ODAs via the outer–inner dynein linkers26.
