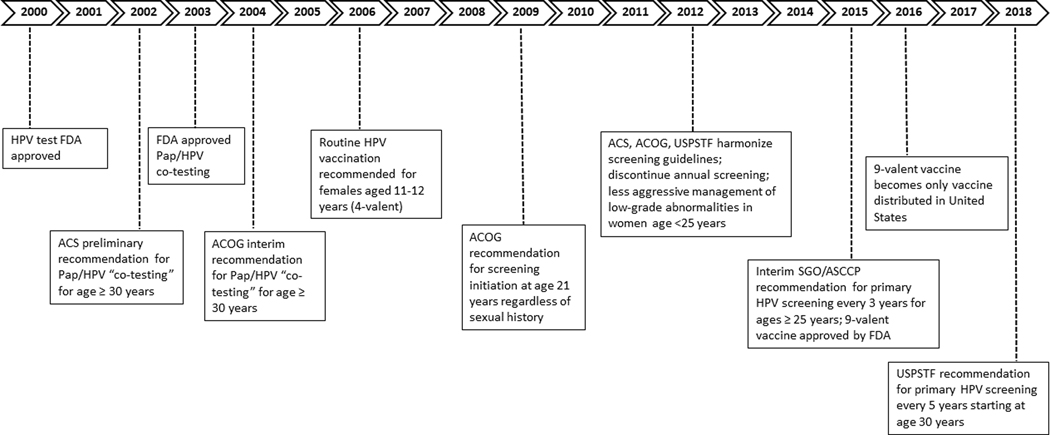
Figure 1. Timeline of relevant recommendations and guidelines in the United States for cervical cancer testing, HPV vaccination, screening, and management.
Abbreviations: HPV = human papillomavirus, FDA = Food and Drug Administration, ACS = American Cancer Society, ACOG = American College of Obstetrics and Gynecology, USPSTF = United States Preventive Services Task Force. This timeline depicts relevant events in the type of testing (Pap/HPV), HPV vaccination, age and frequency of testing, and management of cervical cancers and precancers.