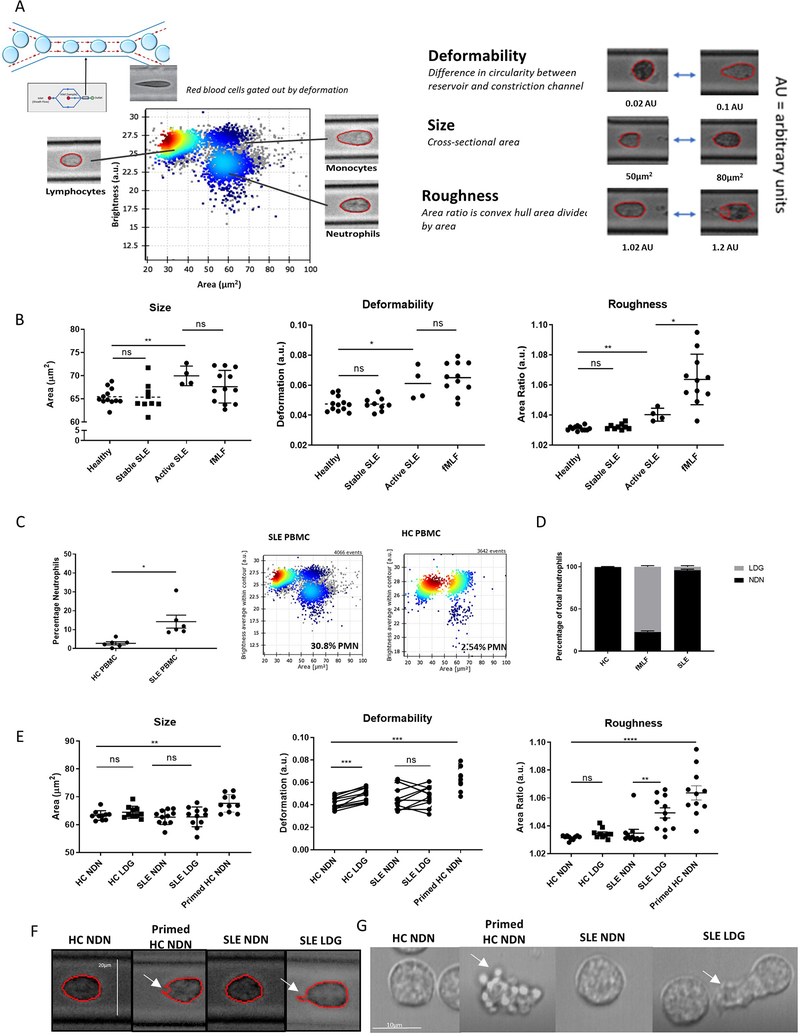
Figure 4. SLE LDGs are biomechanically rougher than other neutrophil subsets by real-time deformability cytometry.
(A) RT-DC was used to biomechanically characterize the shape, size, and deformability of neutrophil subsets. (B) Biomechanical profiling of neutrophils in blood samples obtained from healthy volunteers (n=12), clinically quiescent SLE patients (n=9), and active SLE patients (n=4) by RT-DC. In some instances, 100nM fMLF was used to prime neutrophils within the healthy blood. (C) Percentage of LDGs identified in SLE (n=6) and HC PBMCs (n=6) by RT-DC. (D) LDGs as a percentage of total neutrophils in SLE patients (n=6), HC volunteers (n=6), and fMLF-primed HC blood (n=6). (E) Biomechanical profiling of isolated NDNs and LDGs from HC volunteers (n=11) and clinically quiescent SLE patients (n=11) by RT-DC. Some HC NDNs were primed with fMLF prior to isolation. For each sample analyzed by RT-DC, the median measurement of over 500 neutrophils is graphed and the mean ± SEM for each neutrophil subset is depicted. Autologous unstimulated/primed HC NDNs or autologous SLE NDN/LDGs were compared and significance was established by Wilcoxon matched-pairs signed rank tests. In other comparisons, significance was established by Mann-Whitney U-tests. Significance was set at *p ≤ 0.05, **p≤ 0.01, ns=not significant. (F) Images of NDNs and LDGs captured during RT-DC, representative of >500 images of each neutrophil subset from n=11 SLE patients and n=11 HC volunteers, 10x objective. White arrows identify a concave cell surface feature common in primed HC NDNs and an irregular protrusion in the cell surface common in SLE LDGs. (G) Brightfield microscopy of NDNs and LDGs (n=3). White arrows identify rounded, protruded, membrane features observed in nearly 100% of primed HC NDNs and an irregular protrusion observed in ~30% of SLE LDGs. See supplementary movie files.