Abstract
Engineering microenvironments for accelerated myocardial repair is a challenging goal. Cell therapy has evolved over a few decades to engraft therapeutic cells to replenish lost cardiomyocytes in the left ventricle. However, compelling evidence supports that tailoring specific signals to endogenous cells rather than the direct integration of therapeutic cells could be an attractive strategy for better clinical outcomes. Of many possible routes to instruct endogenous cells, we reviewed recent cases that extracellular matrix (ECM) proteins contribute to enhanced cardiomyocyte proliferation from neonates to adults. In addition, the presence of ECM proteins exerts biophysical regulation in tissue, leading to the control of microenvironments and adaptation for enhanced cardiomyocyte proliferation. Finally, we also summarized recent clinical trials exclusively using ECM proteins, further supporting the notion that engineering ECM proteins would be a critical strategy to enhance myocardial repair without taking any risks or complications of applying therapeutic cardiac cells.
Keywords: extracellular matrix, cardiomyocyte proliferation, myocardial infarction, cardiac repair, acellular therapeutics
Introduction
Post-natal cardiomyocytes (CMs) are terminally differentiated cells in the heart and lack proliferative capacity. A withdrawal from the cell-cycle correlates with multinucleated and polyploid CMs (Derks and Bergmann, 2020). At the time of birth, the neonatal human heart comprises primarily mononucleated CMs and ~30% binucleated CMs, and this proportion of mononucleated and binucleated CMs does not change significantly after birth. The DNA of most nuclei is duplicated to become mononucleated tetraploid in childhood when the cells undergo hypertrophy (Bergmann et al., 2009). The overall arrest in CM division is due to the downregulation of cell cycle regulators (Walsh et al., 2010; Zebrowski et al., 2015).
After myocardial infarction (MI), several strategies to replenish lost CMs have been proposed. Engraftment of exogenous cells to restore the damaged myocardium is an attractive remedy to mitigate the progression of cardiac fibrosis. Exogenous sources include stem cell-derived CMs or cardiac progenitor cells. The first-generation cell therapy employed mesenchymal stem cells (MSCs) or similar derivatives (Perin et al., 2015), and the second-generation cell therapy (Cambria et al., 2017) utilized pluripotent stem cell (PSC)-derived CM (Chong et al., 2014; Shiba et al., 2016; Liu et al., 2018), which showed a certain extent of success for cardiac repair with stem cells. However, a major problem associated with cell therapy includes relatively low retention and integration of delivered cells, where only 10–15% are retained regardless of the source (Hou et al., 2005), and only about 1% of injected cells remained after 1 month (Nguyen et al., 2016). In addition, significant arrhythmia, excessive immunosuppression, and potential teratoma formation are the major roadblocks of applying therapeutic cells toward clinical application (Berry et al., 2019; Bolli and Wysoczynski, 2019). Alternatively, cell therapy with endogenous resources is a preferred strategy. However, the contribution of endogenous cardiac stem cells is proven to be minimal, and 31 associated publications from the Anversa laboratory were retracted (Chien et al., 2019), leading to a pause on a clinical trial involving c-kit+ cardiac stem cells by the National Institutes of Health [National Heart, Lung, and Blood Institute (NHLBI)] as of October 2018. Interestingly, these results can lead to the longstanding notion that it may be possible to achieve cardiac regeneration without physically presenting cells into the injured heart (French and Holmes, 2019). The consistent mismatch between insignificant cellular engraftment and significant functional improvement has led to our assertion that the functional benefits might well be derived from paracrine actions of the transplanted cells (French and Holmes, 2019), which initiated next-generation therapies with cell-free approaches (Cambria et al., 2017). Here, we succinctly summarize extracellular matrix (ECM) proteins directly relevant to or instructing CM proliferation to establish better strategies for enhanced cardiac repair.
Stimulation Signals for Cardiomyocyte Proliferation
Extracellular Matrices Associated With Cardiomyocyte Proliferation
From many recent studies (Frangogiannis, 2019), the composition and mechanical properties of the ECM (Chaudhuri et al., 2020) may play a critical role in inducing the regeneration of the myocardium (Yahalom-Ronen et al., 2015). The following studies are recent investigations on ECM proteins and their roles in CM proliferation.
Fibronectin
Fibronectin is a multidomain, high-molecular-weight glycoprotein, present at low levels in the ECM of the healthy heart. Proteomics analysis of ECM compositions with developmental ages showed that collagen I and III and laminins increase gradually from fetal to adult, while fibronectin decreased with development (Williams et al., 2014). In vivo, fibronectin is strongly upregulated in the heart after MI (Konstandin et al., 2013a). Thus, short-term induction of fibronectin following myocardial injury may be tied to a beneficial role in cardiac repair by CM proliferation. The same group proved that fibronectin promotes CM hypertrophy by nuclear factor of activated T cell (NFAT) in vivo and in vitro, while fibronectin attenuates the activation of physiological growth in vitro (Konstandin et al., 2013b). Co-culture of mammalian embryonic cardiac fibroblast (cFB) and CM can promote CM proliferation, and fibronectin secreted by embryonic mouse cFB plays a pro-proliferative role in this process (Ieda et al., 2009). The mechanism of CM proliferation is only partially attributed to fibronectin or collagen type III that promoted CM proliferation by activating heparin-binding EGF-like GFs via β1 integrin signaling (Ieda et al., 2009). In zebrafish heart regeneration, loss-of-function approaches indicated that high expression of fibronectin does not remuscularize the heart (Wang et al., 2013), but fibronectin is necessary for functional regeneration by mobilizing and integrating CMs into the injured region. As such, cFB and fibronectin need additional players to proliferate CMs for cardiac repair.
Periostin
Periostin is a multimodular protein composed of a signal peptide necessary for secretion, a small cysteine-rich module for the formation of multimers via disulfide bonds, four FAS1 (fasciclin-1) domains interacting with integrins, and a hydrophilic C-terminal region known to interact with other ECM proteins (Kii et al., 2010). Periostin is primarily expressed in the developing heart, but not in healthy adult ventricular myocardium (Snider et al., 2008; Hortells et al., 2020). After acute MI, periostin is re-expressed in the infarct border zones by activated cFBs (Kanisicak et al., 2016). It was reported earlier that periostin can switch differentiated mononucleated CMs into the cell cycle and induce cardiac regeneration with improved myocardial function, as evidenced by an increase in DNA synthesis, aurora B kinase detection, and CM cytokinesis (Kühn et al., 2007). Recently, a report investigated the impact of genetic ablation of periostin in neonatal mice following MI and showed that periostin mediates PI3K/GSK3β/cyclin D1 signaling pathway for myocardial regeneration (Chen et al., 2017). However, this conclusion still remains controversial in adult mice with inducible expression of full-length periostin in that periostin is abundant in the infarcted mouse myocardium in the absence of regeneration (Lorts et al., 2009). Moreover, the periostin-induced cell cycle reentry is mediated in an integrin-dependent manner, which may impact other non-CMs expressing integrins (Kühn et al., 2007).
Agrin
Agrin is a heparan sulfate proteoglycan. It harbors three laminin-globular (LG) domains within its C-terminal region, and the first two LG domains (LG1 and LG2) are sufficient for binding to α-dystroglycan (αDG). Agrin promotes cell cycle reentry in both neonatal and adult mice (Bassat et al., 2017), and a separate study showed that Hippo/Yap signaling is a key signaling mechanism to mediate endogenous CM dedifferentiation and proliferation (Morikawa et al., 2017). In vitro administration of C-terminal agrin from post-natal day 1 (P1) increased CM proliferation (Bassat et al., 2017). The injection of recombinant agrin to the myocardium after MI in juvenile and adult mice also induced CM cell cycle reentry in the healthy myocardium adjacent to the infarcted regions, resulting in reduced scar size and improved cardiac function (Bassat et al., 2017). Although a single administration of agrin promotes cardiac regeneration in adult mice after MI, the degree of CM proliferation observed in this model suggests that additional therapeutic mechanisms are required for functional regeneration of the myocardium (Bassat et al., 2017). In a preclinical porcine model of ischemia reperfusion, local (antegrade) delivery of a single dose of recombinant agrin into the infarcted heart resulted in significant improvement in heart function, infarct size, improved angiogenesis, suppressed inflammatory response, and cell cycle reentry (Baehr et al., 2020). Recent studies reported that binding of agrin to αDG could contribute to enhanced CM proliferation. To expedite such changes, modulating the stiffness of microenvironments could synergistically initiate CM proliferation via dedifferentiation–proliferation–redifferentiation of CMs (Yahalom-Ronen et al., 2015; Wang et al., 2017; Judd et al., 2019).
Slit-2 and Nephronectin
Slit-2 is a neuronal protein and is the only binding partner of αDG with a single LG domain, while two other homologous Slit-1 and Slit-3 are not yet reported to bind to αDG (Wright et al., 2012). Nephronectin is expressed in CMs throughout the heart and is secreted into the cardiac jelly (Patra et al., 2011). From embryonic cFB-derived ECMs, Slit-2 and nephronectin promote CM cytokinesis both in vitro and in vivo (Wu et al., 2020), but not cell cycle entry of post-natal CMs. The authors postulated that Slit-2 and nephronectin may act directly on CM and activate intracellular signaling pathways, such as RhoA (Backer et al., 2018).
Decellularized Extracellular Matrix
Instead of a single ECM component, decellularized zebrafish cardiac ECM (zECM) was intramyocardially injected to treat adult mice after MI (Chen et al., 2016). Given the high regenerative capacity of adult zebrafish hearts, decellularized zECM made from normal or healing hearts can induce mammalian heart regeneration. In a mouse model of acute MI, although a single injection of both normal and healing zECM improved cardiac functional recovery and repair, the healing zECM induced better improvements on heart function. Groups treated with zECM exhibited proliferation of the remaining CMs and multiple cardiac precursor cell populations and reactivation of ErbB2 expression in CMs. NRG1, a mitogen of CMs and a ligand of ErbB2/ErbB4 complex, was detected in zECM but only minimally in murine ECM. The presence of NRG1 in zECM and the reactivation of its receptor ErbB2 in zECM-treated hearts are consistent with the observed proliferation of CMs and improvement of cardiac function. In addition, decellularized porcine myocardial-derived ECM hydrogels were developed (Seif-Naraghi et al., 2013) and showed increases in cardiac muscle and improvements in cardiac function following an injection into the infarct (Christman, 2019). Application of decellularized porcine cardiac ECM to cardiac explant (post-natal day 1) with simultaneous modulation of stiffness using BAPN (3-aminopropionitrile) and ribose (stiffening) (Wang et al., 2020) presented a case that both ECM proteins and mechanical properties of microenvironments are important modulators for cardiac regeneration. Thus, these cases indicate that cardiac ECM-based therapeutics needs to combine with biomechanical modification.
Microenvironmental Contribution to Cardiomyocyte Proliferation
In addition to CM proliferation via cell cycle reentry, heart regeneration requires dedifferentiation, which indirectly initiates proliferation, and the migration of CM to the injured sites, followed by redifferentiation. Clinically, mechanical unloading of diseased hearts can improve adverse remodeling and improve metabolism (Uriel et al., 2018). The stiffness of the ECM in the myocardium increases progressively, which is correlated with CM cell cycle arrest. By modulating the stiffness of polydimethylsiloxane (PDMS) substrates, compliant (5 kPa) substrates promoted dedifferentiation and proliferation of neonatal CMs including a disorganized sarcomere network and conspicuous cell cycle reentry (Yahalom-Ronen et al., 2015). In contrast, rigid (2 MPa) substrates facilitated karyokinesis (nuclear division) leading to binucleation. Thus, the compliant microenvironment could facilitate CM dedifferentiation and proliferation via its effect on the organization of the cytoskeleton (Yahalom-Ronen et al., 2015). In addition to the first report of neonatal (up to 7 days post-partum) cardiac regeneration (Porrello et al., 2011), another recent investigation found that neonatal regeneration sharply declines within 48 h, with hearts of 2-days-old mice responding to amputation with fibrosis, rather than regeneration (Notari et al., 2018). By comparing the global transcriptomes of mouse hearts at P1 and P2, the authors reported that most differentially expressed transcripts encode ECM proteins and structural constituents of the cytoskeleton. Pharmacological inhibition of the cross-linked enzyme LOX (lysyl oxidase) using BAPN rescued the ability of heart regeneration after apical amputation in P3 neonatal mice. On the other hand, stiffer substrates (10 to 50 kPa) were shown to increase CM proliferation and Yap activity in cultures of β-catenin double-knockout CMs (αE-catenin and αT-catenin), indicating that stabilizing cytoskeleton stimulates the nuclear translocation of Yap (Vite et al., 2018). The differences between published works may be attributed to varying experimental techniques including dimensionality, tissue vs. culture conditions, and stiffness range.
Clinical Application of Extracellular Matrix-Based Biomaterials for Cardiac Repair
Here are a few examples for ECM-based biomaterials specifically for cardiac repair. More acellular injectable biomaterials for treating MI are reviewed elsewhere (Hernandez and Christman, 2017; Christman, 2019). In addition, commercially available ECM-based scaffolds for cardiac repair are discussed in the recent reviews therein (Swinehart and Badylak, 2016; Pattar et al., 2019)
CorMatrix is a scaffold derived from small intestinal submucosa (SIS) and is the most widely used SIS-ECM product in cardiovascular surgery, which also recently received Food and Drug Administration (FDA) approval (Mosala Nezhad et al., 2016). CorMatrix ECM cardiac patches were tested in clinical trials (ClinicalTrials.gov identifier: NCT02887768), claiming to promote endogenous cardiac regeneration. However, a study utilizing CorMatrix patches in infants with congenital heart disease did not show evidence of native cardiac tissue ingrowth within 21 months (Nelson et al., 2016). Further complications were reported from other clinical trials with a CorMatrix patch, including patch dehiscence after atrioventricular continuity reconstruction following massive posterior annulus decalcification and mitral valve replacement for mitral stenosis due to dystrophic calcification (Poulin et al., 2013). These results suggest that CorMatrix may elicit eosinophilic inflammation in human patients after implantation, perhaps via α-gal (galactose-α-1,3-galactose) present in the porcine intestine (Mosala Nezhad et al., 2016), which probably supports the notion that completely defined therapeutics would be beneficial to avoid adverse reactions in human patients.
VentriGel is an ECM hydrogel derived from decellularized porcine myocardium (Singelyn et al., 2012; Seif-Naraghi et al., 2013; Hernandez and Christman, 2017) examined in a recently published clinical trial (ClinicalTrials.gov identifier: NCT02305602). The outcomes of the first-in-man trial highlighted the safety and efficacy of the treatment over 6 months (Traverse et al., 2019). VentriGel is a relatively weak hydrogel (Johnson et al., 2011), exhibiting two orders of magnitude lower stiffness than the stiffness (13 Pa of storage modulus at 8 mg/mL) of healthy, normal adult myocardium (around 10–15 kPa; Pandey et al., 2018). While there is yet sufficient evidence for the capability of decellularized hydrogel to promote endogenous cardiac regeneration, the ECM signals of normal healthy myocardium can prove a promising strategy for engineering biomaterials for cardiac repair.
Conclusions and Outlook
From earlier studies treating p38 mitogen-activated protein (MAP) kinase inhibitor (SB203580) for CM mitosis (Engel et al., 2005, 2006), a number of stimulation signals have been identified for CM to reenter cell cycle and to promote [cyclin A2 (Shapiro et al., 2014); a cocktail of CDK1, CDK4, cyclin D1, and B1 (Mohamed et al., 2018); Tbx20 (Xiang et al., 2016); and hypoxia-inducible factor 1α (HIF1α) (Guimarães-Camboa et al., 2015)] or inhibit [Meis1 (Mahmoud et al., 2013) and thyroid hormone (Hirose et al., 2019)] preexisting CM proliferation. Hippo (Heallen et al., 2011; Leach et al., 2017) and NRG1/ErbB4 (D'uva et al., 2015) pathways could be a molecular strategy to promote adult CM proliferation. The Hippo-DGC (dystrophin–glycoprotein complex)-agrin studies identified that viral delivery or a direct injection of CM proliferation agonist could be a viable cardiac repair strategy (Morikawa et al., 2017). More recently, ERBB2-ERK (extracellular signal-regulated kinase)-YAP mechanotransduction signaling was shown to trigger CM mitosis and epithelial-to-mesenchymal (EMT)-like transition toward phenotypic plasticity (Aharonov et al., 2020). Another important consideration is to exploit the metabolic switch from mitochondrial oxidative phosphorylation to glycolysis to induce CM proliferation. Recent studies reported that CM proliferation can be enhanced by inhibiting fatty-acid utilization with deletion of pyruvate dehydrogenase kinase-4 (PDK4) (Cardoso et al., 2020), activating Nrg1/ErbB2 signaling (Honkoop et al., 2019), and activating PPARδ/PDK1/p308Akt/GSK3β/β-catenin-pathway (Magadum et al., 2017).
Translation of technologies to augment the stimulation signals requires a thoughtful examination, especially considering the oncogenic potential of activating growth pathways (Heallen et al., 2019). Another promising strategy of activating CM proliferation is to deliver an intrinsic extracellular factor (e.g., FSTL-1) via an engineered patch to stimulate endogenous repair (Wei et al., 2015). This acellular approach reduces the laborious effort to prepare therapeutic cells, while avoiding potential tumor formation and adverse immune rejection from the patient. However, such an extracellular factor can also potentially stimulate non-CMs. Specific ligands that only allow engagement with CMs are needed to avoid adverse activation of the expansion of cFBs and their differentiation into myofibroblasts (specifically associated with fibrosis) (Fu et al., 2018).
Laminin α chains and several proteoglycans harbor a few tandem arrays of LG domains. Despite the structural similarity between agrin and laminin, binding affinity to αDG and the configuration of a tandem array of LG domains are distinct (Dempsey et al., 2019). This different feature may have conferred the different roles of ECM proteins containing LG domains in CM proliferation and differentiation. Thus, the therapeutic application of LG domain containing ECMs (agrin, laminin, and Slit-2, as depicted in Figure 1) needs to have further specification in their molecular nature and the receptors exclusively expressed in CM (for recent reviews on LG domain containing molecules, see Hohenester, 2019a,b; Yap et al., 2019).
Figure 1.
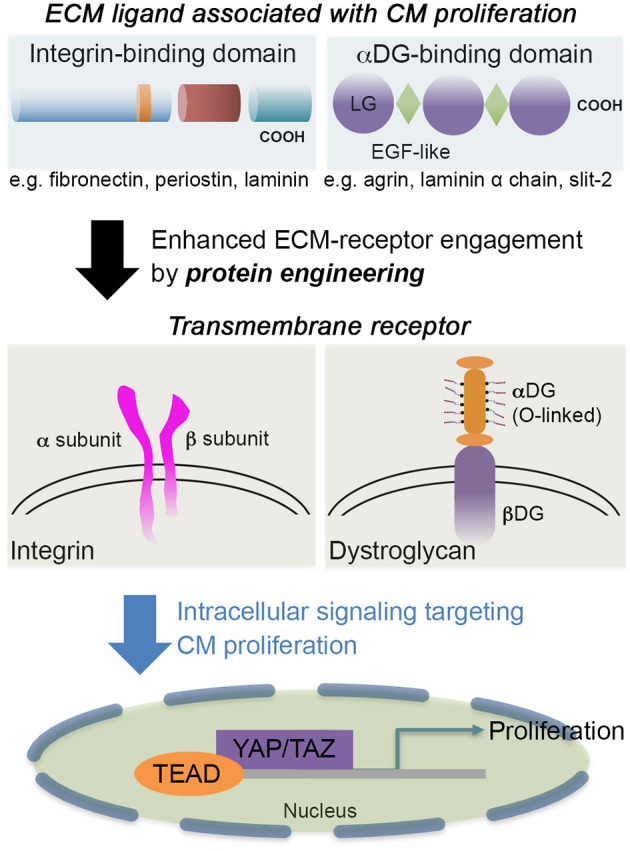
The schematic showcases an example of integrin-binding domain, such as fibronectin, periostin, or laminin; and αDG-binding domain, such as agrin, Slit-2, or laminin α chains. The engagement of extracellular matrix (ECM) proteins via transmembrane receptors [integrin or dystroglycan (DG)] can be tuned by engineering the ECM proteins to further stimulate cardiomyocyte (CM) proliferation and associated pathways without penetrating CMs. Some laminin-globular (LG) domains from laminins are also interactive with integrin (Aumailley, 2013) as well as heparin (Ishihara et al., 2018), so the receptor-ligand integrations are not exclusive. For CM proliferation, active (unphosphorylated) Yap is translocated to the nucleus where the Yap interacts with TEA domain (TEAD) transcription factor to regulate cell proliferation.
ECM proteins that were applied for myocardial regeneration augmented the additional modification to enhance longevity and contribution for remuscularization (e.g., CorMatrix and VentriGel). ECM proteins binding to αDG, agrin, or Slit-2 contributed to CM proliferation (Bigotti et al., 2020), but further mechanistic understanding requires establishing a better strategy for CM proliferation. In addition to providing novel engineering strategies for cardiac repair, more critical analysis of CM proliferation assays and induction of CM proliferation by microRNA, metabolic switch, or small molecule is necessary to inform the field to efficiently reach the goal of myocardial regeneration (Leone and Engel, 2019). A recent phase I clinical trial (ESCORT, NCT02057900) with fibrin and Matrigel composites incorporating human embryonic stem cell (hESC)-derived cardiac progenitor cells (Isl1+) proved the safety of the approach (Menasche et al., 2018; Menasché, 2020), which showed a synergistic contribution to the regeneration of the myocardium without apparent integration of the delivered cardiac cells. Thus, a protein engineering strategy could be a starting point to target a specific receptor and well-defined signal pathways (e.g., Hippo/Yap) (Bassat et al., 2017; Morikawa et al., 2017) (Figure 1). Then, engineering multifactorial, acellular biomaterials with a simple deployment strategy could be a therapeutic goal (Christman, 2019). Ideally, both mechanical compensation and biochemical definition would be necessary. In addition, what levels of complexity we have to address are an important question to answer (Ogle et al., 2016) since a small increase in the ejection fraction of 5–10% in the function of the left ventricle would be a meaningful resolution to mitigate heart failure for patients suffering from post-injury.
Author Contributions
JJ and XF conceived the overall topics of discussion. All authors wrote, read, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was in part supported by the Louisiana Board of Regents Research Competitiveness Subprogram (JJ and XF), NIH 1R15DK122383 (XF), and the Open Access Author Fund from LSU Libraries (JJ). The authors thank Casey Chitwood for helpful discussion.
References
- Aharonov A., Shakked A., Umansky K. B., Savidor A., Genzelinakh A., Kain D., et al. (2020). Erbb2 drives yap activation and emt-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356. 10.1038/s41556-020-00588-4 [DOI] [PubMed] [Google Scholar]
- Aumailley M. (2013). The laminin family. Cell Adh. Migr. 7, 48–55. 10.4161/cam.22826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer S., Lokmane L., Landragin C., Deck M., Garel S., Bloch-Gallego E. (2018). Trio gef mediates rhoa activation downstream of slit2 and coordinates telencephalic wiring. Development 145:dev153692. 10.1242/dev.153692 [DOI] [PubMed] [Google Scholar]
- Baehr A., Umansky K. B., Bassat E., Jurisch V., Klett K., Bozoglu T., et al. (2020). Agrin promotes coordinated therapeutic processes leading to improved cardiac repair in pigs. Circulation 142, 868–881. 10.1161/CIRCULATIONAHA.119.045116 [DOI] [PubMed] [Google Scholar]
- Bassat E., Mutlak Y. E., Genzelinakh A., Shadrin I. Y., Baruch Umansky K., Yifa O., et al. (2017). The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184. 10.1038/nature22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O., Bhardwaj R. D., Bernard S., Zdunek S., Barnabé-Heider F., Walsh S., et al. (2009). Evidence for cardiomyocyte renewal in humans. Science 324, 98–102. 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. L., Zhu W., Tang Y. L., Krishnamurthy P., Ge Y., Cooke J. P., et al. (2019). Convergences of life sciences and engineering in understanding and treating heart failure. Circ. Res. 124, 161–169. 10.1161/CIRCRESAHA.118.314216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigotti M. G., Skeffington K. L., Jones F. P., Caputo M., Brancaccio A. (2020). Agrin-mediated cardiac regeneration: some open questions. Front. Bioeng. Biotech. 8:594 10.3389/fbioe.2020.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., Wysoczynski M. (2019). Human embryonic stem cell-derived cardiomyocytes. Circ. Res. 124, 1157–1159. 10.1161/CIRCRESAHA.119.314869 [DOI] [PubMed] [Google Scholar]
- Cambria E., Pasqualini F. S., Wolint P., Günter J., Steiger J., Bopp A., et al. (2017). Translational cardiac stem cell therapy: advancing from first-generation to next-generation cell types. NPJ Regen. Med. 2:17. 10.1038/s41536-017-0024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso A. C., Lam N. T., Savla J. J., Nakada Y., Pereira A. H. M., Elnwasany A., et al. (2020). Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression. Nat. Metab. 2, 167–178. 10.1038/s42255-020-0169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O., Cooper-White J., Janmey P. A., Mooney D. J., Shenoy V. B. (2020). Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546. 10.1038/s41586-020-2612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C., Wang Z., Missinato M. A., Park D. W., Long D. W., Liu H. J., et al. (2016). Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci. Adv. 2:e1600844. 10.1126/sciadv.1600844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Xie J., Hao H., Lin H., Wang L., Zhang Y., et al. (2017). Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin d1 signalling pathway. Cardiovasc. Res. 113, 620–632. 10.1093/cvr/cvx001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Frisen J., Fritsche-Danielson R., Melton D. A., Murry C. E., Weissman I. L. (2019). Regenerating the field of cardiovascular cell therapy. Nat. Biotech. 37, 232–237. 10.1038/s41587-019-0042-1 [DOI] [PubMed] [Google Scholar]
- Chong J. J. H., Yang X., Don C. W., Minami E., Liu Y.-W., Weyers J. J., et al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277. 10.1038/nature13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman K. L. (2019). Biomaterials for tissue repair. Science 363, 340–341. 10.1126/science.aar2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey C. E., Bigotti M. G., Adams J. C., Brancaccio A. (2019). Analysis of alpha-dystroglycan/lg domain binding modes: investigating protein motifs that regulate the affinity of isolated lg domains. Front. Mol. Biosci. 6:18. 10.3389/fmolb.2019.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks W., Bergmann O. (2020). Polyploidy in cardiomyocytes: roadblock to heart regeneration? Circ. Res. 126, 552–565. 10.1161/CIRCRESAHA.119.315408 [DOI] [PubMed] [Google Scholar]
- D'uva G., Aharonov A., Lauriola M., Kain D., Yahalom-Ronen Y., Carvalho S., et al. (2015). Erbb2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638. 10.1038/ncb3149 [DOI] [PubMed] [Google Scholar]
- Engel F. B., Hsieh P. C., Lee R. T., Keating M. T. (2006). Fgf1/p38 map kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 103, 15546–15551. 10.1073/pnas.0607382103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel F. B., Schebesta M., Duong M. T., Lu G., Ren S., Madwed J. B., et al. (2005). P38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 19, 1175–1187. 10.1101/gad.1306705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis N. G. (2019). The extracellular matrix in ischemic and nonischemic heart failure. Circ. Res. 125, 117–146. 10.1161/CIRCRESAHA.119.311148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French B. A., Holmes J. W. (2019). Implications of scar structure and mechanics for post-infarction cardiac repair and regeneration. Exp. Cell Res. 376, 98–103. 10.1016/j.yexcr.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Fu X., Khalil H., Kanisicak O., Boyer J. G., Vagnozzi R. J., Maliken B. D., et al. (2018). Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Invest. 128, 2127–2143. 10.1172/JCI98215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Camboa N., Stowe J., Aneas I., Sakabe N., Cattaneo P., Henderson L., et al. (2015). Hif1α represses cell stress pathways to allow proliferation of hypoxic fetal cardiomyocytes. Dev. Cell 33, 507–521. 10.1016/j.devcel.2015.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., et al. (2011). Hippo pathway inhibits wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T. R., Kadow Z. A., Kim J. H., Wang J., Martin J. F. (2019). Stimulating cardiogenesis as a treatment for heart failure. Circ. Res. 124, 1647–1657. 10.1161/CIRCRESAHA.118.313573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M. J., Christman K. L. (2017). Designing acellular injectable biomaterial therapeutics for treating myocardial infarction and peripheral artery disease. JACC Basic Transl. Sci. 2, 212–226. 10.1016/j.jacbts.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Payumo A. Y., Cutie S., Hoang A., Zhang H., Guyot R., et al. (2019). Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 364, 184–188. 10.1126/science.aar2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E. (2019a). Laminin g-like domains: dystroglycan-specific lectins. Curr. Opin. Struct. Biol. 56, 56–63. 10.1016/j.sbi.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E. (2019b). Structural biology of laminins. Essays Biochem. 63, 285–295. 10.1042/EBC20180075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkoop H., De Bakker D. E., Aharonov A., Kruse F., Shakked A., Nguyen P. D., et al. (2019). Single-cell analysis uncovers that metabolic reprogramming by erbb2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. Elife 8:e50163. 10.7554/eLife.50163.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortells L., Valiente-Alandi I., Thomas Z. M., Agnew E. J., Schnell D. J., York A. J., et al. (2020). A specialized population of periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation. Proc. Natl. Acad. Sci. U.S.A. 117, 21469–21479. 10.1073/pnas.2009119117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D., Youssef E. A., Brinton T. J., Zhang P., Rogers P., Price E. T., et al. (2005). Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112, I150–I156. 10.1161/CIRCULATIONAHA.104.526749 [DOI] [PubMed] [Google Scholar]
- Ieda M., Tsuchihashi T., Ivey K. N., Ross R. S., Hong T. T., Shaw R. M., et al. (2009). Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev. Cell 16, 233–244. 10.1016/j.devcel.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara J., Ishihara A., Fukunaga K., Sasaki K., White M. J. V., Briquez P. S., et al. (2018). Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 9:2163. 10.1038/s41467-018-04525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. D., Lin S. Y., Christman K. L. (2011). Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology 22:494015. 10.1088/0957-4484/22/49/494015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd J., Lovas J., Huang G. N. (2019). Defined factors to reactivate cell cycle activity in adult mouse cardiomyocytes. Sci. Rep. 9:18830. 10.1038/s41598-019-55027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O., Khalil H., Ivey M. J., Karch J., Maliken B. D., Correll R. N., et al. (2016). Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7:12260. 10.1038/ncomms12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I., Nishiyama T., Li M., Matsumoto K., Saito M., Amizuka N., et al. (2010). Incorporation of tenascin-c into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J. Biol. Chem. 285, 2028–2039. 10.1074/jbc.M109.051961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstandin M. H., Toko H., Gastelum G. M., Quijada P., De La Torre A., Quintana M., et al. (2013a). Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ. Res. 113, 115–125. 10.1161/CIRCRESAHA.113.301152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstandin M. H., Völkers M., Collins B., Quijada P., Quintana M., De La Torre A., et al. (2013b). Fibronectin contributes to pathological cardiac hypertrophy but not physiological growth. Basic Res. Cardiol. 108:375. 10.1007/s00395-013-0375-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn B., Del Monte F., Hajjar R. J., Chang Y. S., Lebeche D., Arab S., et al. (2007). Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 13, 962–969. 10.1038/nm1619 [DOI] [PubMed] [Google Scholar]
- Leach J. P., Heallen T., Zhang M., Rahmani M., Morikawa Y., Hill M. C., et al. (2017). Hippo pathway deficiency reverses systolic heart failure after infarction. Nature 550, 260–264. 10.1038/nature24045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M., Engel F. B. (2019). Advances in heart regeneration based on cardiomyocyte proliferation and regenerative potential of binucleated cardiomyocytes and polyploidization. Clin. Sci. 133, 1229–1253. 10.1042/CS20180560 [DOI] [PubMed] [Google Scholar]
- Liu Y. W., Chen B., Yang X., Fugate J. A., Kalucki F. A., Futakuchi-Tsuchida A., et al. (2018). Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotech. 36, 597–605. 10.1038/nbt.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorts A., Schwanekamp J. A., Elrod J. W., Sargent M. A., Molkentin J. D. (2009). Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ. Res. 104, e1–e7. 10.1161/CIRCRESAHA.108.188649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadum A., Ding Y., He L., Kim T., Vasudevarao M. D., Long Q., et al. (2017). Live cell screening platform identifies pparδ as a regulator of cardiomyocyte proliferation and cardiac repair. Cell Res. 27, 1002–1019. 10.1038/cr.2017.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. I., Kocabas F., Muralidhar S. A., Kimura W., Koura A. S., Thet S., et al. (2013). Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253. 10.1038/nature12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasché P. (2020). Cell therapy with human esc-derived cardiac cells: clinical perspectives. Front. Bioeng. Biotechnol. 8:601560. 10.3389/fbioe.2020.601560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P., Vanneaux V., Hagege A., Bel A., Cholley B., Parouchev A., et al. (2018). Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 71, 429–438. 10.1016/j.jacc.2017.11.047 [DOI] [PubMed] [Google Scholar]
- Mohamed T. M. A., Ang Y. S., Radzinsky E., Zhou P., Huang Y., Elfenbein A., et al. (2018). Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116.e112. 10.1016/j.cell.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y., Heallen T., Leach J., Xiao Y., Martin J. F. (2017). Dystrophin-glycoprotein complex sequesters yap to inhibit cardiomyocyte proliferation. Nature 547, 227–231. 10.1038/nature22979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosala Nezhad Z., Poncelet A., De Kerchove L., Gianello P., Fervaille C., El Khoury G. (2016). Small intestinal submucosa extracellular matrix (cormatrix®) in cardiovascular surgery: a systematic review. Interact. Cardiovasc. Thorac. Surg. 22, 839–850. 10.1093/icvts/ivw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. S., Heider A., Si M. S., Ohye R. G. (2016). Evaluation of explanted cormatrix intracardiac patches in children with congenital heart disease. Ann. Thorac. Surg. 102, 1329–1335. 10.1016/j.athoracsur.2016.03.086 [DOI] [PubMed] [Google Scholar]
- Nguyen P. K., Neofytou E., Rhee J. W., Wu J. C. (2016). Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol. 1, 953–962. 10.1001/jamacardio.2016.2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari M., Ventura-Rubio A., Bedford-Guaus S. J., Jorba I., Mulero L., Navajas D., et al. (2018). The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 4:eaao5553. 10.1126/sciadv.aao5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle B. M., Bursac N., Domian I., Huang N. F., Menasché P., Murry C. E., et al. (2016). Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med. 8:342ps313. 10.1126/scitranslmed.aad2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P., Hawkes W., Hu J., Megone W. V., Gautrot J., Anilkumar N., et al. (2018). Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev. Cell 45:661. 10.1016/j.devcel.2017.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra C., Diehl F., Ferrazzi F., Van Amerongen M. J., Novoyatleva T., Schaefer L., et al. (2011). Nephronectin regulates atrioventricular canal differentiation via bmp4-has2 signaling in zebrafish. Development 138, 4499–4509. 10.1242/dev.067454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattar S. S., Fatehi Hassanabad A., Fedak P. W. M. (2019). Acellular extracellular matrix bioscaffolds for cardiac repair and regeneration. Front. Cell Dev. Biol. 7:63. 10.3389/fcell.2019.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin E. C., Borow K. M., Silva G. V., Demaria A. N., Marroquin O. C., Huang P. P., et al. (2015). A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res. 117, 576–584. 10.1161/CIRCRESAHA.115.306332 [DOI] [PubMed] [Google Scholar]
- Porrello E. R., Mahmoud A. I., Simpson E., Hill J. A., Richardson J. A., Olson E. N., et al. (2011). Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080. 10.1126/science.1200708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin F., Horlick E. M., David T., Woo A., Thavendiranathan P. (2013). 3-dimensional transesophageal echocardiography-guided closure of a gerbode shunt due to cormatrix patch dehiscence. J. Am. Coll. Cardiol. 62:e5. 10.1016/j.jacc.2013.02.090 [DOI] [PubMed] [Google Scholar]
- Seif-Naraghi S. B., Singelyn J. M., Salvatore M. A., Osborn K. G., Wang J. J., Sampat U., et al. (2013). Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 5:173ra125. 10.1126/scitranslmed.3005503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. D., Ranjan A. K., Kawase Y., Cheng R. K., Kara R. J., Bhattacharya R., et al. (2014). Cyclin a2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci. Transl. Med. 6:224ra227. 10.1126/scitranslmed.3007668 [DOI] [PubMed] [Google Scholar]
- Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., et al. (2016). Allogeneic transplantation of ips cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391. 10.1038/nature19815 [DOI] [PubMed] [Google Scholar]
- Singelyn J. M., Sundaramurthy P., Johnson T. D., Schup-Magoffin P. J., Hu D. P., Faulk D. M., et al. (2012). Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J. Am. Coll. Cardiol. 59, 751–763. 10.1016/j.jacc.2011.10.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider P., Hinton R. B., Moreno-Rodriguez R. A., Wang J., Rogers R., Lindsley A., et al. (2008). Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ. Res. 102, 752–760. 10.1161/CIRCRESAHA.107.159517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinehart I. T., Badylak S. F. (2016). Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 245, 351–360. 10.1002/dvdy.24379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse J. H., Henry T. D., Dib N., Patel A. N., Pepine C., Schaer G. L., et al. (2019). First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl. Sci. 4, 659–669. 10.1016/j.jacbts.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriel N., Sayer G., Annamalai S., Kapur N. K., Burkhoff D. (2018). Mechanical unloading in heart failure. J. Am. Coll. Cardiol. 72, 569–580. 10.1016/j.jacc.2018.05.038 [DOI] [PubMed] [Google Scholar]
- Vite A., Zhang C., Yi R., Emms S., Radice G. L. (2018). A-catenin-dependent cytoskeletal tension controls yap activity in the heart. Development 145:dev149823 10.1242/dev.149823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S., Pontén A., Fleischmann B. K., Jovinge S. (2010). Cardiomyocyte cell cycle control and growth estimation in vivo–an analysis based on cardiomyocyte nuclei. Cardiovasc. Res. 86, 365–373. 10.1093/cvr/cvq005 [DOI] [PubMed] [Google Scholar]
- Wang J., Karra R., Dickson A. L., Poss K. D. (2013). Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 382, 427–435. 10.1016/j.ydbio.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. E., Li L., Xia X., Fu W., Liao Q., Lan C., et al. (2017). Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation 136, 834–848. 10.1161/CIRCULATIONAHA.116.024307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Senapati S., Akinbote A., Gnanasambandam B., Park P. S., Senyo S. E. (2020). Microenvironment stiffness requires decellularized cardiac extracellular matrix to promote heart regeneration in the neonatal mouse heart. Acta Biomater. 113, 380–392. 10.1016/j.actbio.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K., Serpooshan V., Hurtado C., Diez-Cuñado M., Zhao M., Maruyama S., et al. (2015). Epicardial fstl1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485. 10.1038/nature15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C., Quinn K. P., Georgakoudi I., Black L. D., III (2014). Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 10, 194–204. 10.1016/j.actbio.2013.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. M., Lyon K. A., Leung H., Leahy D. J., Ma L., Ginty D. D. (2012). Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 76, 931–944. 10.1016/j.neuron.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Jeratsch S., Graumann J., Stainier D. (2020). Modulation of mammalian cardiomyocyte cytokinesis by the extracellular matrix. Circ. Res. 127, 896–907. 10.1161/CIRCRESAHA.119.316303 [DOI] [PubMed] [Google Scholar]
- Xiang F. L., Guo M., Yutzey K. E. (2016). Overexpression of tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation 133, 1081–1092. 10.1161/CIRCULATIONAHA.115.019357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom-Ronen Y., Rajchman D., Sarig R., Geiger B., Tzahor E. (2015). Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife 4:e07455. 10.7554/eLife.07455.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap L., Tay H. G., Nguyen M. T. X., Tjin M. S., Tryggvason K. (2019). Laminins in cellular differentiation. Trends Cell Biol. 29, 987–1000. 10.1016/j.tcb.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Zebrowski D. C., Vergarajauregui S., Wu C. C., Piatkowski T., Becker R., Leone M., et al. (2015). Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. Elife 4:e05563. 10.7554/eLife.05563.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


