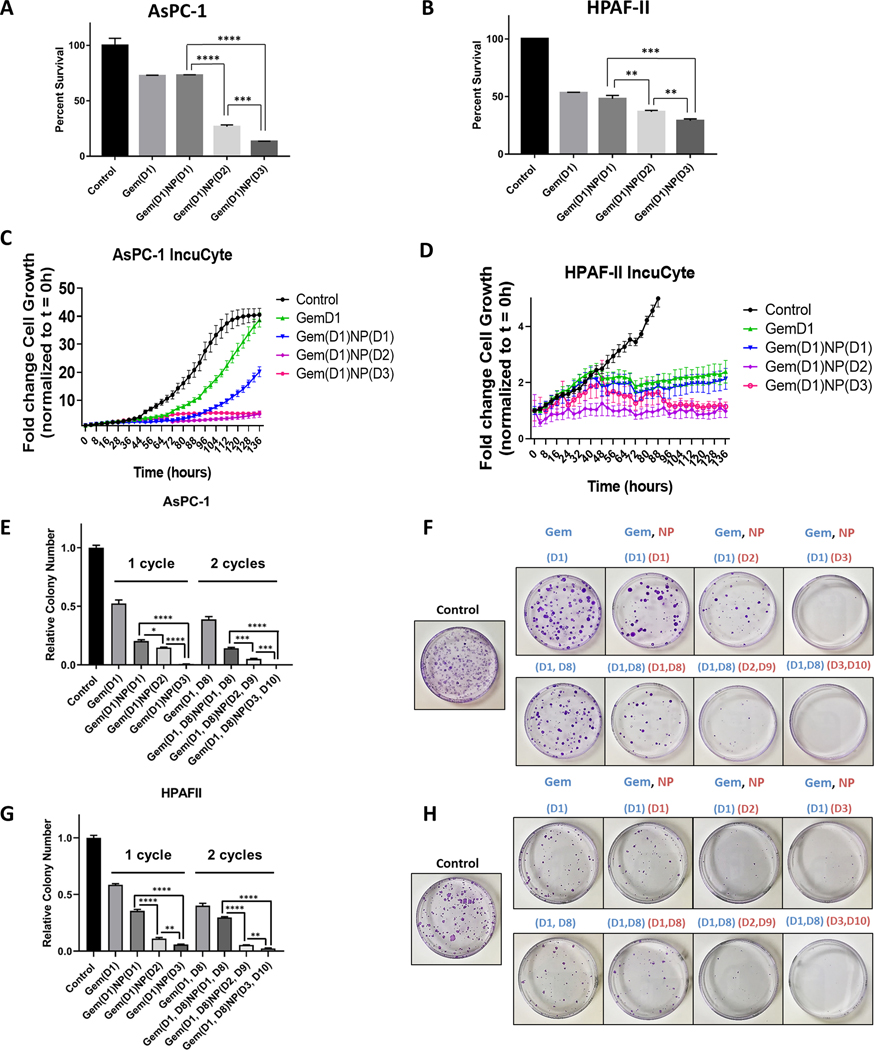
Figure 3.
Gemcitabine delivered prior to NP increases the therapeutic efficacy of the drug combination in vitro. A-B. AsPC-1 and HPAF-II cells were treated with vehicle control or gemcitabine (Gem, 50 nM) for a total of 24 hours with either NP (1 nM) delivered on same day [Gem(D1)NP(D1)], NP added on day 2 [Gem(D1)NP(D2)], or NP treated on day 3 with a 24 hour gap of no treatments [Gem(D1)NP(D3)]. NP and gemcitabine were removed after 24 hours of treatment for each condition. Following 72 hours from the start of treatments, proliferation was measured by the alamarBlue assay. Cell growth displayed as fold-change from control condition (vehicle alone). C-D. AsPC-1 and HPAF-II cells tracked for 136 hours measuring confluence using the IncuCyte live-cell imaging system. Treatment schedules were kept the same as described in the alamarBlue assay (A-B). E-H. Extended time course colony forming assays (3 weeks) with corresponding plate images normalized to the control treatment (vehicle) are shown for AsPC-1 (E-F) and HPAF-II cells (G-H) for the different treatment schedules using 1 or 2 cycles of treatment. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001.