Abstract
Mast cells are tissue resident immune cells that play pivotal roles in initiating and amplifying allergic/anaphylactic reactions in humans. Their activation occurs via multiple mechanisms, which include crosslinking of the immunoglobulin E (IgE)-bound high-affinity IgE receptors (FcεRI) by allergens or antigens (Ag) and the binding of anaphylatoxins such as C3a to its receptor, C3aR. We have previously demonstrated that the Na+/H+ exchanger regulatory factor 1 (NHERF1) promotes C3aR functions in human mast cells. In the current study, we show that NHERF1 regulates mast cell response following FcεRI stimulation. Specifically, intracellular Ca2+ mobilization, activation of the mitogen activated protein (MAP) kinases (ERK1/2 and P38) and production of cytokines (interleukin (IL)-13 and IL-6) following exposure to IgE/Ag were significantly reduced in mast cells from NHERF1+/− mice. In agreement with our in vitro data, mast cell-mediated passive cutaneous anaphylaxis (PCA) and passive systemic anaphylaxis (PSA) were reduced in NHERF1+/− mice and mast cell-deficient Kit W-sh/W-sh mice engrafted with NHERF1+/− mast cells. Mechanistically, the levels of micro (mi)RNAs that regulate mast cell responses, miRNA 155-3p and miRNA 155-5p, were altered in mast cells from NHERF1+/− mice. Moreover, NHERF1 rapidly localized to the nucleus of mast cells following FcεRI stimulation. In summary, our results suggest that the NHERF1 acts as an adapter molecule and promotes IgE/Ag-induced mast cell activation. Further elucidating the mechanisms through which NHERF1 modulates mast cell responses will lend insights into the development of new therapeutic strategies to target mast cells during anaphylaxis or other allergic diseases.
Keywords: Mast cells, Na+/H+ exchanger regulatory factor 1, NHERF1, FcεRI, anaphylaxis, IgE and allergic diseases
INTRODUCTION
Allergic diseases such as anaphylaxis(1), rhinitis(2) and asthma(3) are among the major causes of illness and disability in the United States for all ages. These diseases are caused by an overzealous immune response to allergens or antigens (Ag) in which immunoglobulin E (IgE) and mast cells play critical roles(4, 5). Thus, aggregation of high-affinity IgE receptor (FcεRI) on mast cells by Ag results in rapid histamine release and the generation of lipids mediators and cytokines, which are responsible for the manifestations of the disease. The humanized monoclonal anti-IgE antibody, omalizumab, is one of the best-characterized mast-cell-targeted “bench to bedside” treatment for severe allergic asthma(6, 7). However, omalizumab only works well on a subset of asthmatic patients and has adverse effects including increased risk of heart disease and strokes(8). It is therefore imperative to identify and design novel inhibitors that can target the FcεRI pathway of mast cell activation to prevent and/or treat allergic diseases.
The NHERF1 protein belongs to the NHERF family of PDZ (Post-synaptic density protein 95, Drosophila disc large tumor suppressor and Zonula occludens-1) scaffold proteins that contain two PDZ modules and an ezrin-radixin-moesin (ERM)-binding domain(9, 10). NHERF1 was identified as a regulator of the exchanger NHE3 and hence named Na+/H+ exchanger 3 (NHE3) regulatory factor 1(11, 12). NHERF1 is a major regulator of GPCR signaling(9, 13). Most, if not all, of these GPCRs have a class I PDZ motif S-T-X-Φ, (where “Φ” indicates hydrophobic amino acid and “X” indicates any amino acid) at their carboxyl-terminus (C-terminus) that is recognized by NHERF1. Notably, the interaction between NHERF1 and GPCRs regulate their trafficking, stability, and signaling properties. Specifically, NHERF1 interacts with class I PDZ motif in the C-terminus of GPCRs and anchors them to the membrane and restricts receptor internalization and desensitization(13, 14). Thus, NHERF1 functions as a positive regulator of GPCR signaling. Among the several GPCRs that are regulated by NHERF1, some are involved in regulation of immune cell migration and function including C-X-C chemokine receptor type 2 (CXCR2)(15, 16), the receptor for interleukin (IL)-8 that mediates neutrophil migration to sites of inflammation. NHERF1 also indirectly regulates GPCR signaling in a receptor-independent manner by associating with downstream signaling proteins such as protein kinase A (PKA), phospholipase C (PLC)-β, and protein kinase B (PKB/Akt)(11, 17-20). However, it is currently unclear whether NHERF1 regulates mast cell-FcεRI signaling and/or functions in allergic diseases.
Mast cells express the C3aR(21, 22), a G protein-coupled receptor (GPCR) for the anaphylatoxin C3a. Several reports have demonstrated that the C3aR plays a critical role in mediating allergic responses in conjunction with the FcεRI. Specifically, FcεRI-mediated activation of human mast cells results in the release of proteases that cleave the complement component C3 to generate C3a(23-26). C3a causes mast cell activation by binding to its receptor C3aR and thus potentiates mast cell response. C3aR possesses a class I PDZ motif S-T-X-Φ at its C-terminus. Because NHERF1 is a class I PDZ domain interacting protein that binds to several GPCRs and regulates their signaling, we examined the role of NHERF1 in affecting C3a-induced mast cell activation. Consequently, in a previous report(27), we showed that although NHERF1 does not directly interact with the C3aR, it functions as an adapter molecule and promotes C3a-induced degranulation and chemokine production in human mast cells. Based on these observations and a prior study by Schaffer et al.(26) that showed that C3a amplifies FcεRI-mediated anaphylaxis in vivo, we hypothesized that NHERF1 regulates IgE/Ag induced activation of mast cells. In the current study, we show that NHERF1 promotes IgE/Ag-induced mast cell activation in vivo. Passive cutaneous (PCA) and passive systemic (PSA) anaphylaxis to IgE/Ag are significantly reduced in NHERF1+/− mice as compared to littermate control NHERF1+/+ mice. Bone marrow-derived mast cell (BMMC) engraftment experiments in mast cell-deficient Kit W-sh/W-sh mice revealed that mast cell-specific NHERF1 expression is required for optimal PCA and PSA to IgE/Ag. In vitro experiments with BMMCs cultured from NHERF1+/− mice suggested that NHERF1 promotes IgE/Ag-induced Ca2+ mobilization, activation of the MAP kinases, ERK1/2 and P38 and cytokine (IL-13 and IL-6) production in mast cells. Furthermore, the levels of miRNA 155-3p and miRNA 155-5p, two miRNAs that have been shown to regulate mast cell activation, are differentially regulated in IgE/Ag-stimulated NHERF1+/− BMMC. Interestingly, we observed rapid nuclear localization of NHERF1 following IgE/Ag exposure suggesting its role as an adapter molecule in mast cells. This response however, is abrogated in mast cells from NHERF1+/− mice. Taken together, we demonstrate that NHERF1 regulates FcεRI mediated responses in mast cells and targeting the FcεRI-NHERF1 pathway of mast cell activation may alleviate anaphylaxis and allergic diseases.
MATERIALS AND METHODS
Mice
C57BL/6 (B6, NHERF1+/+), NHERF+/− mice (on the B6 background) and mast cell-deficient Kit W-sh/W-sh mice (fully congenic for the B6 background) were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were bred and housed under specific pathogen-free conditions. Both male and female mice (6-8 weeks old) were used for experiments. The Animal Care and Use Committee at the Michigan State University approved all the animal experiments.
Anaphylaxis assays
PCA:
Mice were injected intradermally (i.d.) with 20 μl of anti-dinitrophenyl (DNP) mouse IgE (Sigma-Aldrich, St. Louis, MO, clone SPE7, 20 ng/mouse) in the left ear and with 20 μl of PBS in the right ear. After 16 h, mice were given 100 μl of antigen (DNP-human serum albumin (HSA), 100 μg/mouse, Sigma-Aldrich) containing 1 % Evans Blue dye in PBS i.v. The animals were euthanized 30 min after the antigen injections; the ears were removed, weighed, dried at 50°C for 2 h and placed in acetone:saline (7:3) for 48 h. The absorbance of the supernatant was measured at 650 nm. For some experiments, following sensitization with the IgE, the ears were injected with the antigen (DNP-HSA). Ear thickness was measured before and after injection of the antigen using a micrometer thickness gauge (Peacock thickness gauge, G-1A) and change in ear thickness was calculated.
PSA:
Mice were injected i.p. with anti-DNP IgE (20 μg/mouse in 100 μl of PBS). The next day, antigen (DNP-HSA, 1 mg/mouse in 100 μl of PBS) was administered i.p. The rectal temperature was recorded at different time points after antigen injection. Mice were euthanized after 2 h and blood and peritoneal lavage fluid were collected by injection of PBS (5 ml) i.p. Serum histamine levels were estimated by LC-MS/MS as described previously(28, 29). The peritoneal lavage fluid was stained with toluidine blue to visually evaluate degranulation.
Cell culture
Mouse BMMC were obtained by flushing bone marrow cells from the femurs of mice and culturing the cells for 4-6 weeks in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% fetal calf serum (FCS), L-glutamine (2 mM), penicillin (100 IU/ml), streptomycin (100 μg/ml) and IL-3 (10 ng/ml) (Peprotech, Cranbury, NJ). After 4 weeks in culture, >95% of these cells were mast cells, as judged by morphology and surface expression of FcεRI and c-Kit (CD117). For some experiments, BMMC were generated by culturing bone marrow cells in IMDM medium supplemented with mouse stem cell factor (SCF, from Peprotech, 10 ng/ml) and IL-3 (10 ng/ml) for 4-6 weeks. Human LAD2 mast cells were maintained in complete StemPro-34 medium supplemented with human stem cell factor (100 ng/ml, Peprotech). Rat basophilic leukemia (RBL-2H3) and HEK-293T cells were obtained from the American Type Culture Collection (Manassas, VA) and were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), L-glutamine (2 mM), penicillin (100 IU/ml) and streptomycin (100 μg/ml). All cell culture reagents were obtained from Invitrogen (Carlsbad, CA).
Flow cytometric analysis of FcεRI expression
BMMC (5 x 105 cells) were washed with cold FACS buffer (PBS containing 2% FBS) and stained with PE-conjugated c-Kit antibody (BD Biosciences, San Jose, CA) and APC-conjugated FcεRI antibody (BD Biosciences) at 4°C for 30 min in the dark. Cells were washed twice with cold FACS buffer and fixed in 250 μl of 1.5% formaldehyde in PBS. Samples were acquired on LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (FlowJo LLC, Ashland, OR).
Ca2+ mobilization
BMMC (5 x 104 cells/well) and RBL-2H3 (2 x 104 cells/well) were sensitized with anti-DNP mouse IgE (clone SPE7, 1 μg/ml) for 12-16 h in cytokine-free media. For some assays, LAD2 cells (5 x 104 cells/well) were sensitized with biotin conjugated IgE (Enzo Life Sciences, Farmingdale, NY, 0.5 μg/ml). The cells were washed, resuspended in media and incubated with the FLIPR® Calcium 6 dye (Molecular Devices, San Jose, CA) for 2 h at 37°C and 5% CO2. Cells were then stimulated with different concentrations of the antigen (DNP-BSA for BMMC and RBL-2H3 cells and Steptavidin for LAD2 cells) and intracellular Ca2+mobilization was measured using a FlexStation® 3 multi-mode plate reader (Molecular devices) with an excitation wavelength of 495 nm and an emission wavelength of 520 nm.
Western blotting
BMMC (4 x 106 cells) were stimulated with DNP-specific IgE (1 μg/ml, 37°C, 5% CO2, 16 h) and DNP-BSA (30 ng/ml) for the indicated time intervals. The cells were washed twice in ice-cold PBS and lysed with RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.5% Sodium-deoxycholate, 0.10% SDS, 50 mM Tris [pH 8.0], 5 mM EDTA, 10 mM NaF, and 10 mM Na-pyrophosphate) containing protease inhibitor cocktail (Roche Applied Sciences; Mannheim, Germany). Proteins (30 μg) were separated on 10% SDS PAGE gels, immunoblotted onto nitrocellulose membranes (GE Healthcare, Chicago, IL), and probed with the phospho-specific antibodies for P38 and ERK1/2 (both from Cell Signaling Technology, 16 h) followed by incubation with LiCor IRDye® 680RD or IRDye® 800CW conjugated secondary antibodies for 2 h in the dark. Blots were washed and imaged using the Li-COR Odyssey Imaging System. Equal loading was confirmed by stripping and re-probing these membranes with P38 and ERK1/2 antibodies (Cell Signaling Technology, Danvers, MA). For assessing the NHERF1 levels in BMMC, LAD2, and RBL-2H3 cells, cells (3 x 106) were lysed in RIPA and proteins (25 μg) were separated on 10% SDS PAGE gels, immunoblotted onto nitrocellulose membranes and probed with the NHERF1 antibody (Abcam, Cambridge, MA, 16 h). The blots were washed and incubated with LiCor IRDye® 800CW conjugated secondary antibody (2 h in dark) and imaged as above. The membranes were stripped and re-probed with the β-Actin antibody (Cell Signaling Technology). Nuclear lysates were prepared from RBL-2H3 cells (3 x 106), NHERF1+/+ BMMCs and NHERF1+/− BMMCs (both at 5 x 106) following stimulation with IgE/Ag using the NE-PER™ nuclear and cytoplasmic extraction reagents (Pierce, ThermoFisher Scientific, Waltham, MA) and probed for NHERF1 expression as described as above. The nuclear protein membranes were stripped and re-probed with the Lamin B1 antibody (Cell Signaling Technology) for endogenous control. Blots were analyzed using ImageJ software.
Cytokine assay
BMMC were incubated with anti-DNP mouse IgE (1 μg/ml) for 12-16 h in cytokine-free media, washed and exposed to varying concentrations of DNP-BSA for 6 h at 37°C and 5% CO2. Cytokines were measured from the supernatants using sandwich ELISA kits for IL-6 and IL-13 (both from Invitrogen) as described by the manufacturer.
Gene and miRNA expression analysis
Ear samples taken from mice were homogenized in liquid N2 using a mortar and pestle. RNA was extracted using TRIzol™ reagent according to the manufacturer’s protocol. RNA (2 μg) was transcribed to cDNA using the high capacity cDNA reverse transcription kit from Applied Biosystems (Foster City, CA). RNA levels (Tnf, Il6 and Il13) were quantified using gene expression assays with TaqMan™ Fast Advanced Master Mix and validated TaqMan™ probes. For miRNA analysis, BMMC were sensitized with IgE (1 μg/ml) for 16 h and stimulated with antigen (DNP-BSA, 30 ng/ml) for 2 h. cDNA was amplified from the mRNA using the TaqMan™ Advanced miRNA cDNA synthesis kit (Applied Biosystems) and miRNA (miR155-3p, miR155-5p, miR126-3p and miR126-5p) expression levels were determined with TaqMan™ Fast Advanced Master Mix and validated miRNA probes from Applied Biosystems.
Lentiviral transduction and NHERF1 knockdown
Generation of lentivirus(27): HEK-293T cells (5 x 106) were transfected with the viral packaging plasmids: p-CMV-VSV-G, pHR’8.2ΔR, and NHERF1 shRNA (Sigma, TRCN0000043736 for NHERF1) or scrambled-sequence control shRNA (Sigma, SCH002) using the Lipofectamine® 2000 reagent (Invitrogen). Viral supernatants were harvested 72 h post-transfection, 0.45 μm sterile-filtered, and concentrated using Vivaspin™ protein concentrators (100 kDa MWCO, GE Healthcare).
Lentiviral transduction(27): LAD2 and RBL-2H3 cells (5 x 106) were washed twice and plated in complete media with hexadimethrine bromide (polybrene, Sigma-Aldrich, 4 μg/mL). Concentrated viral supernatant was then added to cells, centrifuged at 700g for 1 h, and incubated for 8-10 h in 37°C and 5% CO2. Media was changed and cells were exposed to puromycin (2 μg/mL) for selection of stable clones and viable cells were used for Ca2+ mobilization experiments.
Mast cell engraftment in Kit W-sh/W-sh mice
Mast cell engraftment studies were performed as described previously(30). Briefly, BMMCs derived from NHERF1+/+ and NHERF+/− mice were injected i.d. (2 injections per ear; 1 x 106 cells in 20 μL of PBS per injection) into Kit W-sh/W-sh mice 8 weeks before initiating the PCA experiments. Similarly, for PSA assays, Kit W-sh/W-sh mice were injected i.p. with NHERF1+/+ and NHERF+/− BMMC (5 x 106 cells/mouse in 200 μL of PBS) and allowed to engraft for 8 weeks. Mast cell reconstitution in these mice was assessed by toluidine blue staining of the ear sections and peritoneal lavage fluid.
Confocal microscopy
RBL-2H3 cells (1 x 105) were cultured on glass coverslips, sensitized with anti-DNP IgE (1 μg/ml, 16 h) and stimulated with antigen (DNP-BSA, 30 ng/ml). The cells were fixed with 4% paraformaldehyde, and permeabilized with 0.5% saponin for 10 min. The slides were incubated with PBS containing 1% BSA at room temperature for 1 h, washed with PBS with 0.1% Tween (PBST) and incubated with anti-NHERF1 antibody (Abcam, 1:500 dilution) at room temperature for 1 h. Cells were washed with PBST and incubated with Alexa Fluor 555-conjugated anti-rabbit IgG secondary antibody (Invitrogen, 1:300 dilution) for 1 h at room temperature in the dark followed by incubation in 300 μM DAPI (1:1000 dilution). Slides were washed and mounted using the ProLong™ Diamond antifade mounting media (Invitrogen). Confocal images were obtained with the Olympus FV 1000 confocal laser scanning microscope (Olympus America, Center Valley, PA) with a 40X objective and images were analyzed using the ImageJ software.
Statistical analysis
Statistical analyses were performed using GraphPad PRISM software (San Diego, CA) and explained in the figure legends or the results sections. A p-value less than or equal to 0.05 was deemed to be significant.
RESULTS
NHERF1 promotes PCA and PSA to IgE/Ag
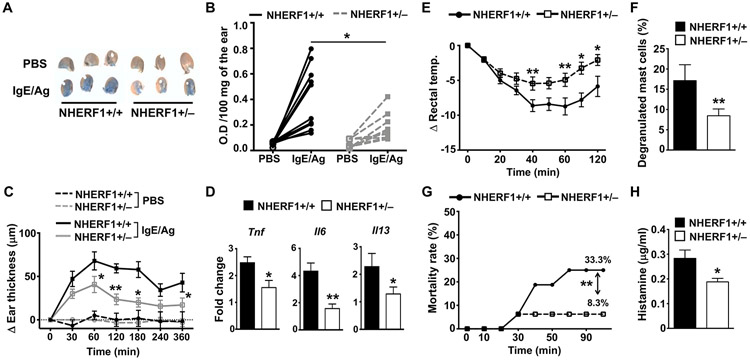
In a prior study, we demonstrated that NHERF1 promotes the activation of mast cells and augments their degranulation and cytokine production to C3a(27). Given that IgE/Ag-dependent PCA in mice is enhanced by the C3a-C3aR pathway(26), we hypothesized that NHERF1 would also modulate the anaphylaxis reaction to IgE/Ag. To determine the role of NHERF1 in regulating mast cell response in vivo, we first performed PCA assays to IgE/Ag. We used NHERF1+/− mice for our experiments because NHERF1−/− mice suffer from several abnormalities such as reduced bone mineral density(31), defective kidney and colon functions(32), and hydrocephaly(33, 34). In addition, female NHERF1−/− mice have high mortality rates and die very early after birth; and those that survive have multiple bone fractures and must be euthanized within 4-6 weeks of age. We injected the ears of NHERF1+/+ and NHERF1+/− mice with anti-DNP IgE and 18 h later i.v. administered Ag (DNP-BSA) to these mice. As expected, we observed a significant reduction in PCA (as indicated by the O.D. reading after extraction of the Evans blue dye from the ears) to IgE/Ag in NHERF1+/− mice as compared to the littermate control NHERF1+/+ mice (Figs. 1A and 1B). We also measured ear thickness and analyzed the gene expression of pro-inflammatory cytokines (Tnf, Il6 and Il13) secreted by mast cells in different cohorts of mice. As indicated in Figs. 1C and 1D, NHERF1+/− mice showed significant reduction in ear thickness and decreased Tnf, Il6, and Il13 expression levels in the ears following IgE/Ag exposure.
Figure 1: NHERF1+/− mice show decreased PCA and PSA to IgE/Ag.
(A-D) NHERF1+/+ and NHERF1+/− mice were injected with anti-DNP IgE (20 ng, left ear) and PBS (right ear). DNP-HSA (Ag, 100 μg) in 1% Evan’s Blue in PBS was administered i.v in these mice after 18 h. Mice were culled after 30 min, the ears were weighed, the dye was extracted, and the absorbance was measured at 650 nm. (A). Representative pictures of the ears from 3 different mice for each cohort are shown. (B) The line graph shows O.D. of the extracted dye per 100 mg of the ear tissue after the PBS or the Ag injections. (C, D) Mice were exposed to IgE/Ag, and (C) ear thickness was measured at different time points after Ag injection, and the change in ear thickness is shown. (D) Mice were sacrificed and the ear samples were collected. Bar graph shows the fold change (2−ΔΔCt) in the mRNAs of TNF, IL-6, and IL-13 relative to GAPDH levels. (E-H) NHERF1+/+ and NHERF1+/− mice were sensitized with anti-DNP IgE (20 μg, i.p.) for 16 h. and were injected with DNP-HSA (Ag, 1 mg) i.p. (E) The rectal temperature was measured before and after Ag treatment at the indicated time points (0-120 min) and the change in temperature is shown. (F) Bar graph shows numbers of degranulated mast cells in the peritoneal lavage fluid. (F) Some mice died following the DNP-HSA injection, the mortality rate was estimated and is shown in the graph. (H) Histamine levels in the serum of mice is shown. Data shown are mean ± S.E. from 2-3 experiments (n=5-10 mice/group). Statistical significance was determined by unpaired Student’s t-test. * p<0.05 and ** p<0.01.
To investigate the role of NHERF1 in mediating PSA, we injected NHERF1+/+ and NHERF1+/− mice with IgE i.p. and 18 h later exposed the mice to the Ag, DNP-BSA (i.p.). This model of activation stimulates mast cells rapidly to release histamine, which in turn, induces anaphylaxis resulting in a significant decrease in body temperature. Our data suggests that NHERF1 potentiates PSA responses to IgE/Ag in vivo. Specifically, we observed a drastic decrease in body temperature in NHERF1+/+ mice within 40 min after Ag injection, which recovered with time (Fig. 1E). This change in body temperature was significantly attenuated in NHERF1+/− mice. In addition, there was a significant decrease in the number of degranulated mast cells in the peritoneum of NHERF1+/− mice (Fig. 1F). Some NHERF1+/+ mice (n=5) died due to the PSA reaction induced by IgE/Ag exposure; however, as shown in Fig. 1G, the mortality rate was lower in NHERF1+/− mice when compared to control NHERF1+/+ mice. The histamine levels in the serum were also diminished in NHERF1+/− mice, suggesting that these mice were protected from PSA (Fig. 1H). Collectively, our data suggest that NHERF1 promotes both IgE/Ag-induced PCA and PSA in vivo.
Mast cell numbers and their maturation in the skin and peritoneum of NHERF1+/− mice
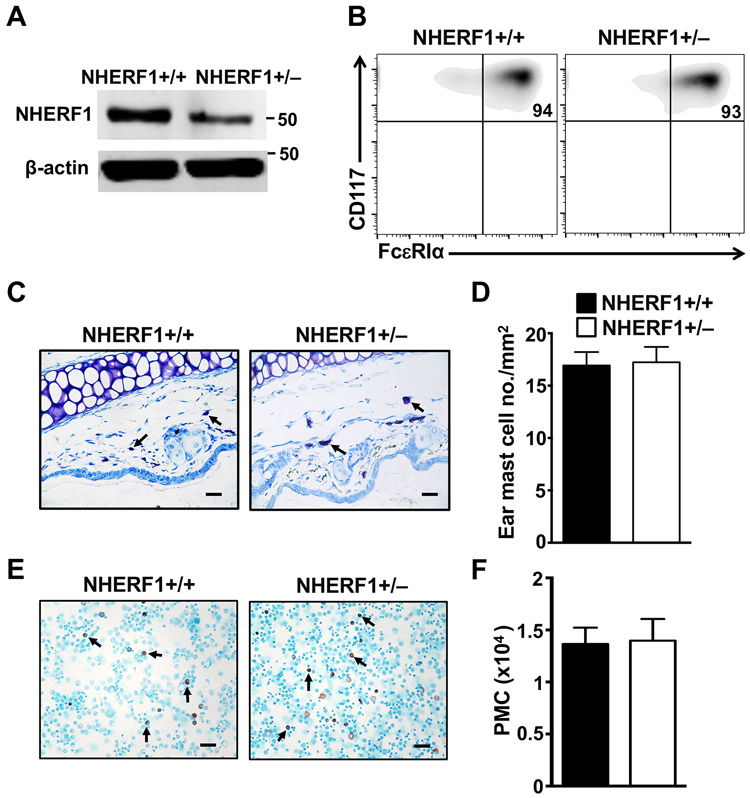
Since the anaphylaxis response to IgE/Ag is primarily mediated by mast cells, we next assessed mast cell numbers and maturation in the NHERF1+/− mice. In addition, to determine if IgE receptor expression levels are altered in NHERF1+/− mast cells, we cultured mast cells ex vivo and performed flow cytometric analysis on these cells. Specifically, bone marrow cells from NHERF1+/+ and NHERF1+/− mice were cultured in the presence of IL-3 for 4 weeks to obtain BMMC. Mast cells from both mice expanded in culture with similar growth characteristics (data not shown). We examined NHERF1 expression levels in BMMC by western blotting. As expected, NHERF1 expression was reduced by ~50% in NHERF1+/− BMMC (Fig. 2A). Flow cytometry analysis indicated that there was no difference in FcεRIα and CD117 (c-Kit) expression levels between NHERF1+/− and NHERF1+/+ BMMC and >90% of these cells expressed both these markers suggesting that most of the ex vivo cultured cells were mast cells (Fig. 2B). Furthermore, toluidine blue staining of skin sections and Alcian blue/Safranin staining of peritoneal lavage cytospin slides revealed that both the number of mast cells and their maturation, based on the morphology revealed by our histological methods, appeared identical between the NHERF1+/− and NHERF1+/+ mice (Figs 2C-2E).
Figure 2: Characterization of mast cells from NHERF1+/− mice.
(A) Blots show expression levels of NHERF1 protein in BMMCs from NHERF1+/+ and NHERF1+/− mice. The blots were stripped and re-probed with β-actin for loading control. (B) Flow cytometric plots show the expression of FcεRI and CD117 on NHERF1+/+ and NHERF1+/− BMMCs. (C) Representative pictures showing mast cells (bold arrows) in the toluidine blue stained sections of the ear tissue are shown. (D) The mast cells were counted and total numbers of mast cells per mm2 in the ears of mice are plotted. (E-F) The peritoneal lavage from mice were stained with Alcian blue and Safranin stains. (E) Representative images showing peritoneal mast cells (PMC, bold arrows) and (F) total numbers of PMC in lavage fluid are shown. Scale bar = 20 μM. Data shown are representative of n=3-4 mice in each group.
NHERF1 enhances activation of BMMCs to IgE/Ag in vitro
C3a enhances IgE-dependent anaphylaxis(24, 26). So, the observed decrease in the PCA and PSA experiments (Fig. 1) could be either due to the role of NHERF1 in regulating mast cell activation through the IgE receptor directly, or via the C3a pathway. To dissect this, we performed in vitro assays with BMMCs as they do not express the C3aR(25). Intracellular Ca2+ mobilization and MAP kinase activation represent early events of mast cell activation, whereas the delayed phase consists of the production and release of cytokines such as IL-13 and IL-6. NHERF1+/+ and NHERF1+/− BMMCs cultured in IL-3 were exposed to IgE/Ag and intracellular Ca2+ mobilization and MAP kinase activation assays were performed. IgE-sensitized NHERF1+/+ BMMC showed a dose-dependent increase in intracellular Ca2+ mobilization (as indicated by a change in fluorescence intensity following labeling with a Ca2+ sensitive fluorescent dye) following Ag exposure (Fig. 3A). However, this response was significantly attenuated in NHERF1+/− BMMCs. Mast cell stimulation via FcεRI results in activation of MAP kinases such as P38 and ERK1/2. Accordingly, we observed an increase in phospho-ERK1/2 and phospho-P38 levels when NHERF1+/+ BMMCs were exposed to IgE/Ag. In contrast, NHERF1+/− BMMCs showed reduced activation of these MAP kinases after Ag stimulation (Fig. 3B-D). Consistently, IgE-exposed NHERF1+/− BMMCs produced significantly lower levels of IL-13 and IL-6 when compared to control NHERF1+/+ BMMCs over the range of Ag concentrations (0-100 ng/ml) tested (Figs. 4A and 4B). Additionally, we performed mast cell activation experiments with NHERF1+/+ and NHERF1+/− BMMC cultured in the presence of both SCF and IL-3. Our data demonstrates that intracellular Ca2+ mobilization and cytokine (IL-13 and IL-6) production were significantly decreased in BMMC that had reduced expression levels of NHERF1 (Supplemental figure 1). Taken together, these data suggested that NHERF1 regulated both the early and delayed phases of mast cell activation via FcεRI.
Figure 3: Ca2+ influx and MAP kinase (P-38 and ERK1/2) activation is reduced in NHERF1+/− BMMCs.
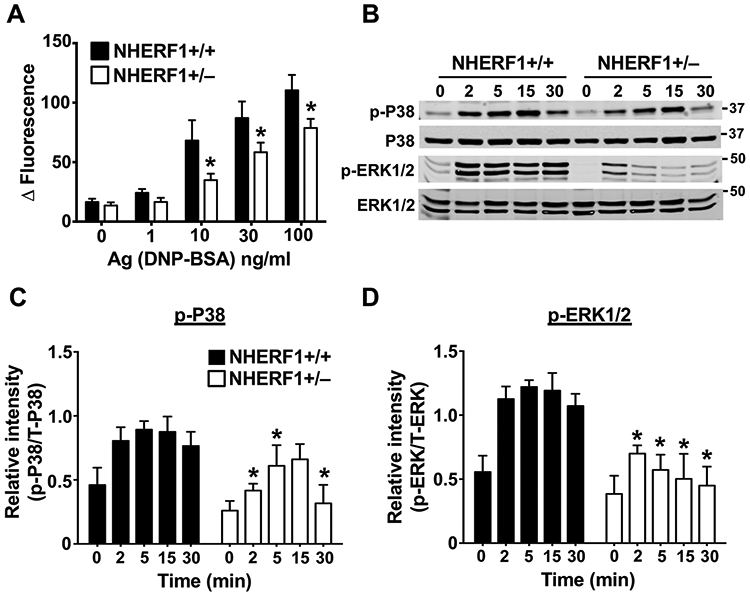
(A) IgE-sensitized NHERF1+/+ and NHERF1+/− BMMCs were loaded with Calcium 6 dye reagent and stimulated with indicated concentrations of DNP-BSA (Ag). Bar graphs show change in fluorescence intensities following Ag exposure. (B-D) NHERF1+/+ and NHERF1+/− BMMCs were pretreated with IgE and stimulated with DNP-BSA (30 ng/mL) for the indicated time intervals. Western blotting was performed on cell lysates with the appropriate primary and secondary antibodies. (B) Representative blots from three similar experiments are shown. (C, D) Bar graphs represent relative intensities of analyzed phospho-protein bands normalized to the respective total proteins. Data are mean ± SEM from three independent experiments. Statistical significance was determined by unpaired Student’s t-test. * p <0.05.
Figure 4. NHERF1+/− BMMCs showed decreased IL-6 and IL-13 production and altered miRNA 155 levels following exposure to IgE/Ag.
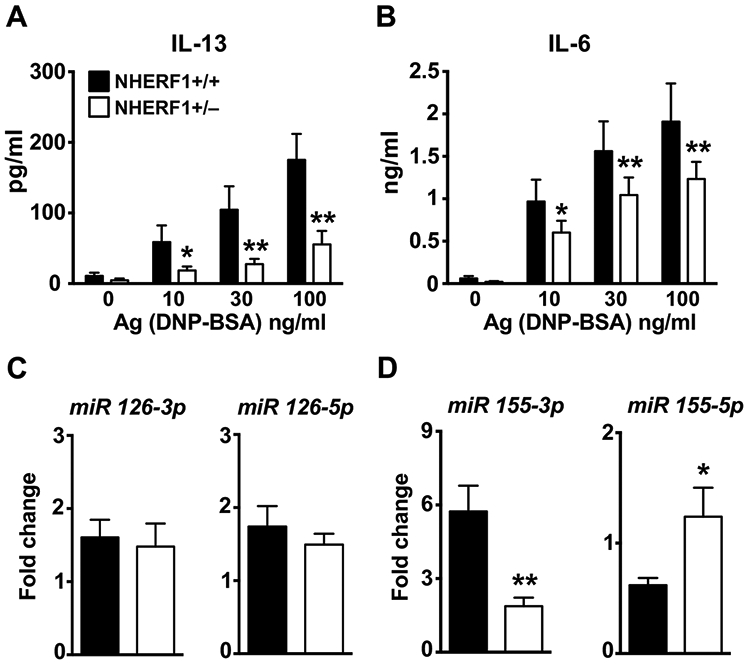
NHERF1+/+ and NHERF1+/− BMMCs were pretreated with IgE and were stimulated with DNP-BSA for 6h. (A) IL-13 and (B) IL-6 production was determined by ELISA. (C-D) BMMCs were stimulated with IgE and DNP-BSA for 1 h and miRNA expression of (C) 126-3p and 126-5p and (D) 155-3p and 155-5p was analyzed by real-time PCR. Values are plotted as fold change (2−ΔΔCt) normalized to GAPDH levels. Data are mean ± S.E. from 3 experiments (n=5 mice/group). Statistical significance was determined by unpaired Student’s t-test. * indicates p<0.05, and ** indicates p<0.01.
Micro (mi)RNAs are small noncoding RNAs that can bind to the 3’ untranslated regions of multiple targets to degrade mRNA and regulate gene expression. Thus, miRNAs are involved in many developmental and pathologic processes. Previous reports have shown that miRNA-126(35) and miRNA-155(36, 37) play critical roles in regulating mast activation. To test whether NHERF1 modulated these miRNAs, we examined the expression levels of miRNA-126 and miRNA-155 in NHERF1+/+ and NHERF1+/− BMMCs following exposure to IgE/Ag. Interestingly, there was no change in miRNA-126 (both 3p and 5p) (Fig. 4C), however miRNA-155-3p was substantially reduced while miR-155-5p was elevated in NHERF1+/− BMMCs (Fig. 4D).
Mast cell-specific NHERF1 expression plays a critical role in mediating PCA and PSA
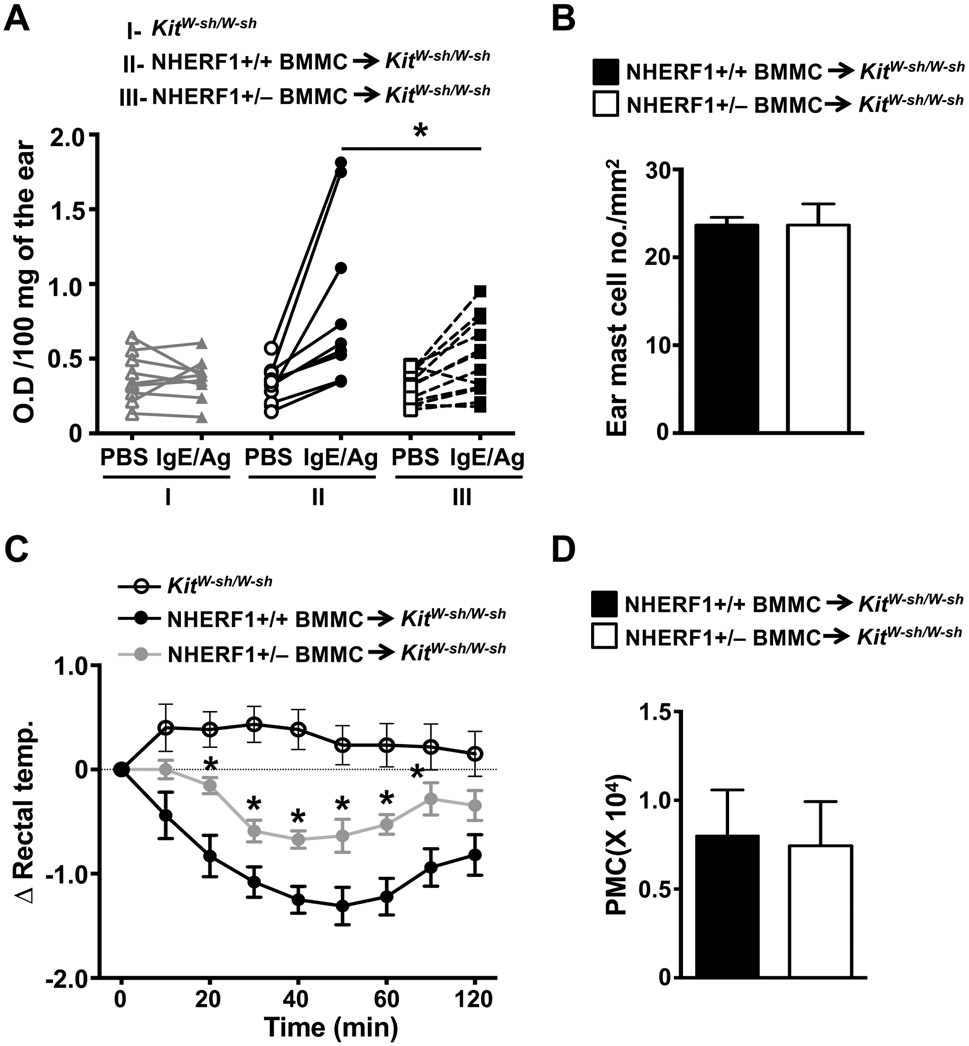
Our data in Fig. 1 shows that NHERF1 regulates PCA and PSA to IgE/Ag. To affirm that NHERF1 expression on mast cells contributed to the reduced anaphylaxis response observed in the NHERF1+/− animals, we adopted the well-established mast cell engraftment approach (“knock-in”) in mast cell-deficient Kit W-sh/W-sh mice. Specifically, we reconstituted Kit W-sh/W-sh mice with in vitro cultured NHERF1+/+ or NHERF1+/− BMMCs via i.d. injections in the ears (for PCA) or i.p injections (for PSA) and performed the anaphylaxis assays 8-12 weeks after engraftment. As expected, Kit W-sh/W-sh mice engrafted with NHERF1+/+ BMMCs exhibited PCA (Fig. 5A) and PSA (Fig. 5C) to IgE/Ag. However, these responses were significantly attenuated in mice reconstituted with NHERF1+/− BMMCs. Kit W-sh/W-sh mice that were not engrafted with BMMCs did not show any notable PCA or PSA reaction to IgE/Ag (Figs. 5A and 5C). Notably, there was no difference in the numbers of mast cells in the ears or the peritoneal lavage fluid in different cohorts of mice (Figs. 5B and 5D) indicating that NHERF1 does not affect engraftment of mast cells into these tissues. These data suggest that NHERF1 expression in mast cells does indeed regulate PCA and PSA to IgE/Ag in vivo.
Figure 5: PCA and PSA responses are reduced in mast cell deficient Kit W-sh/W-sh mice engrafted with NHERF1+/− BMMCs.
Kit W-sh/W-sh mice (I), Kit W-sh/W-sh mice engrafted with NHERF1+/+ BMMCs (II) or Kit W-sh/W-sh mice reconstituted with NHERF1+/− BMMCs (III) were subjected to (A, B) PCA and (C, D) PSA assays 8 weeks post engraftment. (A) The line graph shows O.D. of the extracted Evan’s Blue dye per 100 mg of the ear tissue from each mouse after PBS or IgE/Ag injections. (B) The ear sections from different cohorts of mice were stained with toluidine blue and the number of mast cells in the ears was counted. (C) Graph shows change in rectal temperature at different time points following the exposure to IgE/Ag in different groups of mice. (D) The peritoneal lavages of mice were stained with toluidine blue and numbers of mast cells were counted and plotted. Data shown are mean ± S.E. from 3 experiments (a total of n=5-11 mice/group). Statistical significance was determined by unpaired Student’s t-test. * p <0.05.
NHERF1 amplifies IgE/Ag-induced Ca2+ mobilization in human and rat mast cells that express FcεRI
The human and rat mast cell lines LAD2 and RBL-2H3 respectively, have been extensively used as tools to study the signaling mechanisms of FcεRI. To determine if NHERF1 also regulates IgE/Ag-induced responses in human and rat mast cells, we knockdown the expression of NHERF1 in both LAD2 and RBL-2H3 cells using the lentiviral transduction approach. We achieved ~70% knockdown of NHERF1 in these cells, as shown in the western blots in Figs. 6A and 6C. In agreement with the data obtained with BMMC (Fig. 3A), silencing NHERF1 expression in LAD2 and RBL-2H3 cells resulted in attenuation of Ca2+ mobilization (Figs. 6B and 6D) suggesting that NHERF1 regulates IgE/Ag-induced responses in both human and rat mast cell lines that express FcεRI.
Figure 6: Knockdown of NHERF1 in LAD2 and RBL-2H3 cells results in reduced intracellular Ca2+ mobilization.
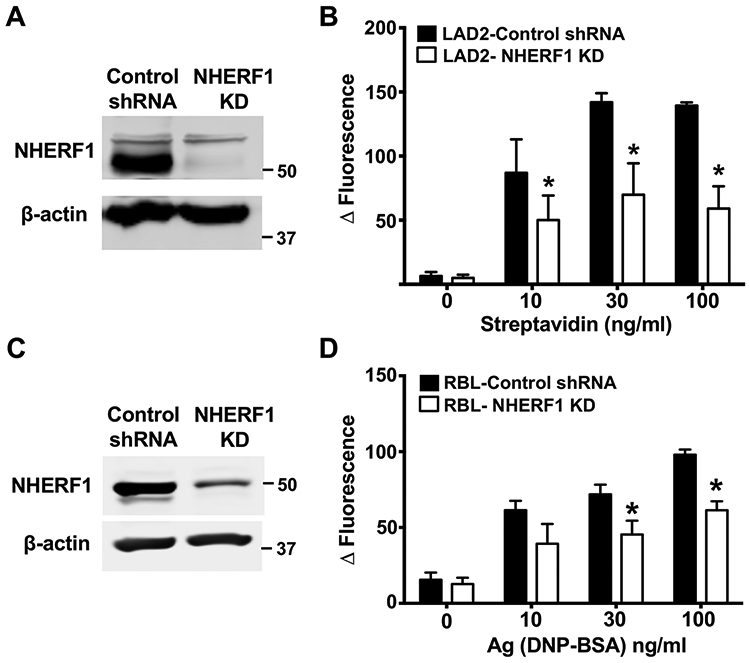
LAD2 human mast cells and RBL-2H3 cells were transduced with scrambled shRNA lentivirus (control) or a shRNA lentivirus that targets NHERF1 (NHERF1 KD). (A and C) Western blotting was performed to determine NHERF1 levels in control and NHERF1 silenced LAD2 and RBL cells. Representative images of the blots are shown. The blots were probed with β-actin as loading control. (B) Control or NHERF1 knock down LAD2 cells (LAD2-NHERF1 KD) were pretreated with biotin-IgE (10 ng/mL, 24 h) and stimulated with different concentrations streptavidin and intracellular Ca2+ mobilization assay was performed. (D) Control or NHERF1 shRNA transduced RBL-2H3 cells (RBL-NHERF1 KD) were exposed to DNP-specific IgE and DNP-BSA (Ag) and intracellular Ca2+ mobilization assay was performed. Data are mean ± SEM of three independent experiments. Statistical significance was determined by unpaired Student’s t-test. * p <0.05.
Subcellular localization of NHERF1 following IgE/Ag stimulation of mast cells
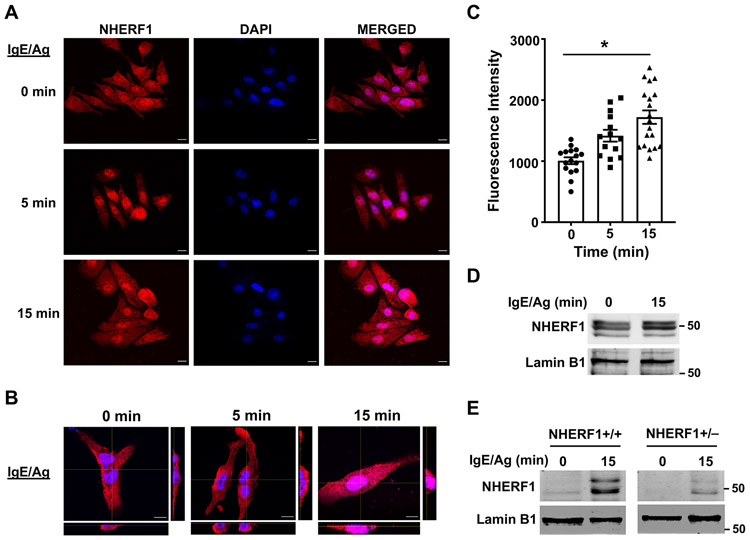
NHERF1 is predominantly a cytosolic protein; however, nuclear expression of NHERF1 has been described in certain cancer cells. Additionally, it has been suggested that upregulation of NHERF1 in the nucleus is a prognostic marker for breast and colon cancers(38, 39). To determine if the localization of NHERF1 in mast cells is altered following activation, we exposed RBL-2H3 cells to IgE/Ag and analyzed NHERF1 expression in the cytoplasmic and nuclear compartments by confocal microscopy. NHERF1 is expressed both in the cytoplasm (especially, near the plasma membrane) and nucleus at resting conditions (Fig. 7A). However, 15 mins following stimulation with IgE/Ag, NHERF1 is significantly upregulated in the nucleus of RBL-2H3 cells (Fig. 7A-7C); the cytoplasmic expression levels are however unaltered (Fig. 7A and 7B). We also prepared nuclear lysates from IgE/Ag-stimulated RBL-2H3 cells and determined NHERF1 levels by western blotting. We observed increased NHERF1 protein levels in the nucleus of samples stimulated with IgE/Ag (Fig. 7D). To further confirm this observation, we determined nuclear NHERF1 levels in IgE/Ag-stimulated NHERF1+/+ or NHERF1+/− BMMCs (Fig. 7E). In agreement with our data with RBL-2H3 cells, nuclear NHERF1 level was upregulated in NHERF1+/+ BMMC stimulated with IgE/Ag. Interestingly, NHERF1+/− BMMCs showed a reduction of NHERF1 in the nucleus (fold increase over control was 1.5±0.07 for NHERF1+/+ BMMC vs 1.1±0.07 for NHERF1+/− BMMCs).
Figure 7: NHERF1 localizes to the nucleus following IgE/Ag stimulation.
RBL-2H3 cells were plated on poly-L-lysine coated slides, pre-treated with IgE and stimulated with Ag at different time points. Cells were fixed with 4% paraformaldehyde and stained with fluorescent antibodies to detect NHERF1. (A) Representative images of NHERF1 expression (red) and DAPI staining (nucleus, blue) are shown. (B) Z-stack confocal microscopy images with cross-sectional views taken at 100 x magnification show localization of NHERF1 (red) after Ag stimulation. Scale bar = 10 μm. (C) Bar graph shows mean fluorescence intensities in the red channel that co-localized with the blue color intensities corresponding to NHERF1 expression in the nucleus. Data represent mean ± S.E. from 3 experiments with 14-19 cells analyzed in each group. Statistical analysis was done using unpaired t-test. * p<0.05. (D, E) RBL-2H3 cells (D) and NHERF1+/+ and NHERF1+/− BMMCs (E) were stimulated with IgE/Ag for 15 min and NHERF1 protein levels in the nucleus was determined by western blotting. A representative image of the blot is shown. The blots were stripped and re-probed with lamin B1 as loading control.
DISCUSSION
NHERF1 is a member of a large family of PDZ domain binding proteins, and its role in regulating GPCR function via interaction with the PDZ domains is well characterized. Specifically, NHERF1 tethers GPCRs onto the membrane and inhibits their internalization; thus, NHERF1 generally acts as a positive regulator of GPCR signaling(9, 13). We have previously shown that NHERF1 promotes C3aR responses in mast cells independent of its PDZ domain binding function but rather by acting as an adapter molecule to promote downstream signaling(27). In the present study, we show that NHERF1 enhances FcεRI signaling in mast cells. We used multiple strategies including in vitro assays with BMMC from NHERF1+/− mice and lentiviral shRNA-mediated knockdown of NHERF1 protein in rat and human mast cell lines, and in vivo mast cell-induced anaphylaxis assays using NHERF1+/− mice to clearly demonstrate that NHERF1 regulates both the early (Ca2+ mobilization and MAP kinase signaling) and delayed (cytokine production) phases of mast cell activation. Importantly, we have used NHERF1+/− mice for most of the experiments in this study, suggesting that even a partial reduction of NHERF1 levels is enough to regulate mast cell activation.
NHERF1 has been shown to function as an adapter/scaffolding protein that regulates the responses of different signaling and Ca2+ channel proteins such as phosphatase and tensin homologue (PTEN)(40), PLC-β(18), PKB (Akt)(19), transient receptor potential (TRP)C4/5(41) and TRPV6(42) in several cell types. In the present study, we demonstrate that NHERF1 promotes Ca2+ mobilization and phosphorylation of P38 and ERK1/2 following IgE/Ag activation of mast cells. While PTEN(43), PLC-β(44), and TRP(45, 46) proteins have been implicated to play a role in modulating FcεRI signaling, whether NHERF1 modulates mast cell responses by regulating the functions of these proteins is currently unknown. We observed a dramatic reduction in in vitro cytokine (IL-6 and IL-13) production in NHERF1+/− BMMC. The defect in cytokine production could be a result of reduced proximal signaling due to decreased Ca2+ influx or aberrant activation of P38 or ERK1/2 in these cells. However, since NHERF1 can also interact with cytoskeletal elements such as ERM family of proteins and α-actinin(9, 47, 48), it is possible that the reduction in cytokine production observed in NHERF1+/− BMMC is due to abrogation of the NHERF1-ERM /NHERF1-α-actinin interactions resulting in diminished exocytosis and release of the cytokines.
The in vitro findings with BMMC corroborate with our in vivo results where we observed a significant reduction in mast cell-mediated cutaneous (PCA) and systemic (PSA) anaphylaxis in NHERF1+/− mice or mast cell-deficient Kit W-sh/W-sh mice engrafted with NHERF1+/−BMMCs. It is important to note that mast cell maturation and numbers in the skin and peritoneal tissues were not altered in these mice suggesting that the reduced anaphylaxis response was because of the lack of NHERF1 expression in mast cells and not due to reduced mast cell maturation or numbers. It has been proposed that mast cell activation via FcεRI results in the generation of C3a that can amplify IgE/Ag-induced response via the C3aR expressed on these cells(24-26). Since NHERF1 attenuated C3a-induced human mast cell activation and given that C3a amplifies the IgE/Ag response, it is possible that the reduction in PCA and PSA response is actually a reflection of NHERF1 modulating the C3a-C3aR pathway that is downstream of FcεRI. However, NHERF1 also promotes IgE/Ag induced activation of BMMC, which does not express the C3aR(25). Thus, NHERF1 does affect mast cell activation following FcεRI stimulation independent of the C3a-C3aR pathway. In support of this notion, we also observed a significant reduction in intracellular Ca2+ mobilization in NHERF1 silenced RBL-2H3 cells that lack the C3aR. Consequently, NHERF1 regulation of anaphylactic responses in vivo may occur via its effects on either FcεRI or C3aR or both of these receptors.
Previous reports have suggested that NHERF1 is post-translationally modified, affecting its functions in several cell types. NHERF1 possesses two PDZ domains (PDZ1 and PDZ2) and an ERM binding domain. Hall et al. showed that G-protein receptor kinase (GRK) 6 interacts with PDZ1 motif of NHERF1 to promote its phosphorylation at Ser289(49). Given that GRK6 associates with and phosphorylates NHERF1, it is possible that this modification may provide a mechanism for FcεRI-induced mast cell activation. Interestingly, PKC also phosphorylates NHERF1 at Ser162 (PDZ2 domain) and Ser337/338 (C-terminus)(50). It is noteworthy that FcεRI -induced mast cell responses are dependent on PKC activation(51, 52) and PKC binds to NHERF1 through the PDZ domain(53). Thus FcεRI, PKC, and NHERF1 can form a signaling complex to promote phosphorylation of downstream signaling proteins to support mast cell activation. Whether these or other mechanisms regulates NHERF1 functions in mast cells remains to be determined.
An interesting and surprising finding of the present study was that NHERF1 inversely regulates miRNA 155-3p and 155-5p levels after IgE/Ag stimulation. These miRNAs have been shown to regulate mast cell responses such as degranulation and cytokine production. Specifically, in vitro mast cell degranulation and cytokine production and in vivo PCA responses via IgE are enhanced in miRNA 155−/− mice(37) that lack miRNA 155-5p, suggesting that miRNA 155-5p inhibits mast cell response. Consistent with these observations, miRNA 155-5p is significantly upregulated in NHERF1+/− BMMCs. In another study, Qayum et al.(36) showed that IL-10 amplified mast cells responses in vitro and in vivo, and that miRNA 155-3p contributes to the increase in mast cell activation. We also observed a similar pattern in the current study, where miRNA 155-3p was substantially reduced in NHERF1+/− BMMCs. These data suggest that NHERF1 appears to regulate mast cell responses, at least in part, by altering the levels of miRNA 155-5p and miRNA 155-3p. It is currently unknown how NHERF1 regulates these miRNAs. In addition, there is no literature till date that describes the role of NHERF1 in controlling miRNA levels in any cell type. Thus, the finding that NHERF1 regulates miRNA 155-3p and 155-5p in mast cells is novel and warrants further investigation. Further experiments using miRNA 155-knock mice or miRNA 155-deficient BMMC need be performed to conclusively determine the role of NHERF1 in regulating mast cell activation via miRNA 155.
To determine if NHERF1 modulated responses in human and rat mast cell lines, we knocked down its expression in RBL-2H3 and LAD2 cells. Consistent with our observation with mouse BMMCs, reduction in NHERF1 levels resulted in an attenuated Ca2+ mobilization induced by IgE/Ag in RBL-2H3 and LAD2 cells. Given that we have used NHERF1+/− BMMCs and NHERF1 knockdown-RBL-2H3 and -LAD2 cells that still express some NHERF1, it is possible that a complete deletion of NHERF1 would result in a more drastic phenotype. However, we were not able to obtain BMMCs from NHERF1−/− mice to test this contention; the female NHERF1−/− mice die very early after birth and the bone marrow cells from male NHERF1−/− mice did not proliferate well ex vivo (data not shown). Another alternative would be to use peritoneal mast cells from male NHERF1−/− mice. Recent reports(54, 55) however suggest that there are sex-based differences in IgE/Ag mast cell responses with females showing enhanced responses as compared to males. Thus NHERF1−/− mast cells from both male and female mice are required to effectively delineate the role of NHERF1. Generation of mice with NHERF1 deletion specifically in mast cells or using conditional deletion models where NHERF1 can be deleted in mast cells following maturation will hopefully shed more light into the role of NHERF1 in regulating mast cell responses.
Our data reveals that NHERF1 is rapidly accumulated in the nucleus following IgE/Ag stimulation of mast cells. Additionally, we observed reduced nuclear localization of NHERF1 in NHERF1+/− BMMC suggesting that the decreased mast cell responses observed in the NHERF1+/− BMMC may be because of the aberrant nuclear localization of NHERF1. It is however, also possible that the decreased nuclear localization in the NHERF1+/− BMMC is because of the lower expression levels of NHERF1 in these cells. Interestingly, the cytoplasmic expression levels of NHERF1 were unaffected. Nuclear expression of NHERF1 is found in malignant breast cancer cells(38). A recent study by Du et al.(56) suggested that a mutation or truncation in the PDZ1 domain of NHERF1 was responsible for its nuclear localization. IgE/Ag stimulation of mast cells may alter the PDZ1 domain of NHERF1, resulting in its accumulation in the nucleus. It is currently unclear if NHERF1 translocates from the cytoplasm to the nucleus, or if its release from the nucleus is halted. However, because the cytoplasmic NHERF1 levels are unaltered, it is tempting to speculate that the nuclear export is affected. In conclusion, we have identified NHERF1 as a novel adapter molecule that regulates mast cell activation via FcεRI. This study thus uncovers a novel mechanism for the regulation of FcεRI signaling in mast cells that can be exploited further for development of new therapies for mast cell-dependent pathologic conditions such as anaphylaxis, asthma, and other allergic disorders.
Supplementary Material
Key Points:
NHERF1 promotes mast cell activation following FcεRI stimulation in vitro
NHERF1 regulates IgE mediated mast cell-dependent anaphylactic reactions in vivo
NHERF1 localized to the mast cell nucleus following IgE/Ag stimulation
ACKNOWLEDGMENTS
We thank Drs. Arnold Kirshenbaum and Dean Metcalfe at The Laboratory of Allergic Disease at the National Institutes of Health (Bethesda, MD) for providing the LAD2 mast cells. We thank the histology, flow cytometry and confocal microscopy core facilities at Michigan State University for their assistance in the current study. We thank Dr. Rupali Das for critical review of our manuscript and insightful suggestions. We also thank Brianna Callahan for editing the manuscript.
This work was supported by a grant from the National Institutes of Health (NIH) 5R00HL121073 and the Michigan State University start-up funds to H.S. C.J.O. and C.Y. were supported by an Undergraduate Research Scholarship from Michigan State University.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- 1.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, and Boyle RJ. 2017. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J Allergy Clin Immunol Pract 5: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeConde AS, and Soler ZM. 2016. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 30: 134–139. [DOI] [PubMed] [Google Scholar]
- 3.Papi A, Brightling C, Pedersen SE, and Reddel HK. 2018. Asthma. Lancet 391: 783–800. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, and Tsai M. 2012. IgE and mast cells in allergic disease. Nat Med 18: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito H, Ishizaka T, and Ishizaka K. 2013. Mast cells and IgE: from history to today. Allergol Int 62: 3–12. [DOI] [PubMed] [Google Scholar]
- 6.Corren J, Kavati A, Ortiz B, Colby JA, Ruiz K, Maiese BA, Cadarette SM, and Panettieri RA Jr. 2017. Efficacy and safety of omalizumab in children and adolescents with moderate-to-severe asthma: A systematic literature review. Allergy Asthma Proc 38: 250–263. [DOI] [PubMed] [Google Scholar]
- 7.Logsdon SL, and Oettgen HC. 2015. Anti-IgE therapy: clinical utility and mechanistic insights. Curr Top Microbiol Immunol 388: 39–61. [DOI] [PubMed] [Google Scholar]
- 8.Iribarren C, Rahmaoui A, Long AA, Szefler SJ, Bradley MS, Carrigan G, Eisner MD, Chen H, Omachi TA, Farkouh ME, and Rothman KJ. 2017. Cardiovascular and cerebrovascular events among patients receiving omalizumab: Results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol 139: 1489–1495 e1485. [DOI] [PubMed] [Google Scholar]
- 9.Broadbent D, Ahmadzai MM, Kammala AK, Yang C, Occhiuto C, Das R, and Subramanian H. 2017. Roles of NHERF Family of PDZ-Binding Proteins in Regulating GPCR Functions. Adv Immunol 136: 353–385. [DOI] [PubMed] [Google Scholar]
- 10.Vaquero J, Nguyen Ho-Bouldoires TH, Claperon A, and Fouassier L. 2017. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: from signaling regulation to clinical relevance. Oncogene 36: 3067–3079. [DOI] [PubMed] [Google Scholar]
- 11.Weinman EJ, Steplock D, Wang Y, and Shenolikar S. 1995. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J Clin Invest 95: 2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinman EJ, Cunningham R, and Shenolikar S. 2005. NHERF and regulation of the renal sodium-hydrogen exchanger NHE3. Pflugers Arch 450: 137–144. [DOI] [PubMed] [Google Scholar]
- 13.Ardura JA, and Friedman PA. 2011. Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol Rev 63: 882–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Yang Y, Abou-Samra AB, and Friedman PA. 2009. NHERF1 regulates parathyroid hormone receptor desensitization: interference with beta-arrestin binding. Mol Pharmacol 75: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Wang S, Farooq SM, Castelvetere MP, Hou Y, Gao JL, Navarro JV, Oupicky D, Sun F, and Li C. 2012. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J Biol Chem 287: 5744–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Y, Wu Y, Farooq SM, Guan X, Wang S, Liu Y, Oblak JJ, Holcomb J, Jiang Y, Strieter RM, Lasley RD, Arbab AS, Sun F, Li C, and Yang Z. 2015. A critical role of CXCR2 PDZ-mediated interactions in endothelial progenitor cell homing and angiogenesis. Stem Cell Res 14: 133–143. [DOI] [PubMed] [Google Scholar]
- 17.Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, and Shenolikar S. 2006. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol 68: 491–505. [DOI] [PubMed] [Google Scholar]
- 18.Suh PG, Hwang JI, Ryu SH, Donowitz M, and Kim JH. 2001. The roles of PDZ-containing proteins in PLC-beta-mediated signaling. Biochem Biophys Res Commun 288: 1–7. [DOI] [PubMed] [Google Scholar]
- 19.Song GJ, Leslie KL, Barrick S, Mamonova T, Fitzpatrick JM, Drombosky KW, Peyser N, Wang B, Pellegrini M, Bauer PM, Friedman PA, Mierke DF, and Bisello A. 2015. Phosphorylation of ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) by Akt promotes stability and mitogenic function of S-phase kinase-associated protein-2 (Skp2). J Biol Chem 290: 2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie KL, Song GJ, Barrick S, Wehbi VL, Vilardaga JP, Bauer PM, and Bisello A. 2013. Ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) and nuclear factor-kappaB (NF-kappaB): a feed-forward loop for systemic and vascular inflammation. J Biol Chem 288: 36426–36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesha RT, Berla Thangam E, Zaidi AK, and Ali H. 2005. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol 42: 581–587. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q, Subramanian H, Gupta K, and Ali H. 2011. Regulation of C3a receptor signaling in human mast cells by G protein coupled receptor kinases. PLoS One 6: e22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, and Schwartz LB. 2008. Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J Immunol 180: 6307–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz LB, Kawahara MS, Hugli TE, Vik D, Fearon DT, and Austen KF. 1983. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J Immunol 130: 1891–1895. [PubMed] [Google Scholar]
- 25.Ali H 2010. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett 128: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, Tsai M, Kalesnikoff J, and Galli SJ. 2013. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol 131: 541–548 e541-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian H, Gupta K, and Ali H. 2012. Roles for NHERF1 and NHERF2 on the regulation of C3a receptor signaling in human mast cells. PLoS One 7: e51355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Occhiuto CJ, Kammala AK, Yang C, Nellutla R, Garcia M, Gomez G, and Subramanian H. 2019. Store-Operated Calcium Entry via STIM1 Contributes to MRGPRX2 Induced Mast Cell Functions. Front Immunol 10: 3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chimalakonda KC, Pang E, Weaver JL, Howard KE, Patel V, and Boyne MT 2nd. 2015. Development and validation of a liquid-chromatography tandem mass spectrometry method to determine in vitro and in vivo histamine release. J Pharm Biomed Anal 102: 494–499. [DOI] [PubMed] [Google Scholar]
- 30.Gaudenzio N, Sibilano R, Starkl P, Tsai M, Galli SJ, and Reber LL. 2015. Analyzing the Functions of Mast Cells In Vivo Using 'Mast Cell Knock-in' Mice. J Vis Exp: e52753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, and Weinman EJ. 2002. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci U S A 99: 11470–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lunnemann M, Rottinghaus I, Krabbenhoft A, Engelhardt R, Rausch B, Weinman EJ, Donowitz M, Hubbard A, Kocher O, de Jonge HR, Hogema BM, and Seidler U. 2009. Defective jejunal and colonic salt absorption and alteredNa(+)/H (+) exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflugers Arch 457: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treat AC, Wheeler DS, Stolz DB, Tsang M, Friedman PA, and Romero G. 2016. The PDZ Protein Na+/H+ Exchanger Regulatory Factor-1 (NHERF1) Regulates Planar Cell Polarity and Motile Cilia Organization. PLoS One 11: e0153144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgescu MM, Yell P, Mobley BC, Shang P, Georgescu T, Wang SH, Canoll P, Hatanpaa KJ, White CL 3rd, and Raisanen JM. 2015. NHERF1/EBP50 is an organizer of polarity structures and a diagnostic marker in ependymoma. Acta Neuropathol Commun 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao Y, Wang S, Gao Y, Zhang W, Jin H, Yang Y, and Li J. 2018. MicroRNA-126 accelerates IgE-mediated mast cell degranulation associated with the PI3K/Akt signaling pathway by promoting Ca(2+) influx. Exp Ther Med 16: 2763–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qayum AA, Paranjape A, Abebayehu D, Kolawole EM, Haque TT, McLeod JJ, Spence AJ, Caslin HL, Taruselli MT, Chumanevich AP, Baker B, Oskeritzian CA, and Ryan JJ. 2016. IL-10-Induced miR-155 Targets SOCS1 To Enhance IgE-Mediated Mast Cell Function. J Immunol 196: 4457–4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biethahn K, Orinska Z, Vigorito E, Goyeneche-Patino DA, Mirghomizadeh F, Foger N, and Bulfone-Paus S. 2014. miRNA-155 controls mast cell activation by regulating the PI3Kgamma pathway and anaphylaxis in a mouse model. Allergy 69: 752–762. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Du G, Yu Z, Si Y, Martin TA, He J, Cheng S, and Jiang WG. 2017. A Novel NHERF1 Mutation in Human Breast Cancer and Effects on Malignant Progression. Anticancer Res 37: 67–73. [DOI] [PubMed] [Google Scholar]
- 39.Mangia A, Saponaro C, Malfettone A, Bisceglie D, Bellizzi A, Asselti M, Popescu O, Reshkin SJ, Paradiso A, and Simone G. 2012. Involvement of nuclear NHERF1 in colorectal cancer progression. Oncol Rep 28: 889–894. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi Y, Morales FC, Kreimann EL, and Georgescu MM. 2006. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J 25: 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storch U, Forst AL, Pardatscher F, Erdogmus S, Philipp M, Gregoritza M, Mederos YSM, and Gudermann T. 2017. Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc Natl Acad Sci U S A 114: E37–E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohmer C, Palmada M, Kenngott C, Lindner R, Klaus F, Laufer J, and Lang F. 2007. Regulation of the epithelial calcium channel TRPV6 by the serum and glucocorticoid-inducible kinase isoforms SGK1 and SGK3. FEBS Lett 581: 5586–5590. [DOI] [PubMed] [Google Scholar]
- 43.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, and Rivera J. 2006. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol 176: 5167–5171. [DOI] [PubMed] [Google Scholar]
- 44.Xiao W, Kashiwakura J, Hong H, Yasudo H, Ando T, Maeda-Yamamoto M, Wu D, Kawakami Y, and Kawakami T. 2011. Phospholipase C-beta3 regulates FcvarepsilonRI-mediated mast cell activation by recruiting the protein phosphatase SHP-1. Immunity 34: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wajdner HE, Farrington J, Barnard C, Peachell PT, Schnackenberg CG, Marino JP Jr., Xu X, Affleck K, Begg M, and Seward EP. 2017. Orai and TRPC channel characterization in FcepsilonRI-mediated calcium signaling and mediator secretion in human mast cells. Physiol Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solis-Lopez A, Kriebs U, Marx A, Mannebach S, Liedtke WB, Caterina MJ, Freichel M, and Tsvilovskyy VV. 2017. Analysis of TRPV channel activation by stimulation of FCepsilonRI and MRGPR receptors in mouse peritoneal mast cells. PLoS One 12: e0171366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finnerty CM, Chambers D, Ingraffea J, Faber HR, Karplus PA, and Bretscher A. 2004. The EBP50-moesin interaction involves a binding site regulated by direct masking on the FERM domain. J Cell Sci 117: 1547–1552. [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Zheng J, Wang Q, Song R, Liu H, Meng R, Tao T, Si Y, Jiang W, and He J. 2016. NHERF1 regulates actin cytoskeleton organization through modulation of alpha-actinin-4 stability. FASEB J 30: 578–589. [DOI] [PubMed] [Google Scholar]
- 49.Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, and Lefkowitz RJ. 1999. G protein-coupled receptor kinase 6A phosphorylates the Na(+)/H(+) exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem 274: 24328–24334. [DOI] [PubMed] [Google Scholar]
- 50.Raghuram V, Hormuth H, and Foskett JK. 2003. A kinase-regulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc Natl Acad Sci U S A 100: 9620–9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lessmann E, Leitges M, and Huber M. 2006. A redundant role for PKC-epsilon in mast cell signaling and effector function. Int Immunol 18: 767–773. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Graham C, Parravicini V, Brown MJ, Rivera J, and Shaw S. 2001. Protein kinase C theta is expressed in mast cells and is functionally involved in Fcepsilon receptor I signaling. J Leukoc Biol 69: 831–840. [PubMed] [Google Scholar]
- 53.Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, and Donowitz M. 2003. Ca2+-dependent inhibition of NHE3 requires PKC alpha which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol 285: C1527–1536. [DOI] [PubMed] [Google Scholar]
- 54.Mackey E, Ayyadurai S, Pohl CS, S DC, Li Y, and Moeser AJ. 2016. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackey E, Thelen KM, Bali V, Fardisi M, Trowbridge M, Jordan CL, and Moeser AJ. 2020. Perinatal androgens organize sex differences in mast cells and attenuate anaphylaxis severity into adulthood. Proc Natl Acad Sci U S A 117: 23751–23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du G, Gu Y, Hao C, Yuan Z, He J, Jiang WG, and Cheng S. 2016. The cellular distribution of Na+/H+ exchanger regulatory factor 1 is determined by the PDZ-I domain and regulates the malignant progression of breast cancer. Oncotarget 7: 29440–29453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.