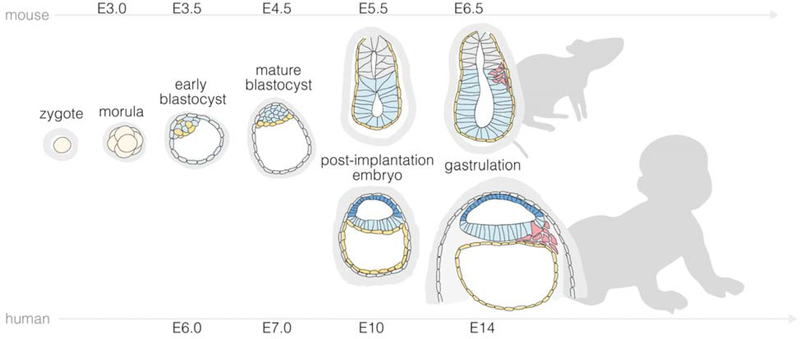
Figure 1. Overview of mouse and human development from pre-implantation to the onset of gastrulation.
Prior to implantation, both mouse and human embryos undergo cell divisions culminating in the development of a blastocyst, comprising an outer trophectoderm (TE) layer and an inner cell mass (ICM) that further segregates into epiblast (EPI) and primitive endoderm (PE; hypoblast in humans). The timing of blastocyst implantation differs between mice and humans (E5 in mouse and E7 in human). Furthermore, morphogenesis and lineage developments during peri-implantation mouse and human development show distinct features. Mouse development from E5 - E6.5 leads to the formation of a cup-shaped EPI juxtaposed with TE-derived extraembryonic ectoderm (ExE), enclosing the pro-amniotic cavity. Concurrently, the PE forms the visceral endoderm (VE) that envelops both EPI and ExE. In contrast, soon after human blastocyst implantation, while the EPI undergoes lumenogenesis to form the pro-amniotic cavity, EPI cells adjacent to polar TE cells become specified into the amniotic ectoderm (AM), with remaining pluripotent EPI cells forming the embryonic disc. By E6.5 for mice and E14 for humans, gastrulation is initiated in the posterior EPI compartment. Mouse primordial germ cells (PGCs) emerge at the boundary between posterior EPI and ExE at the onset of gastrulation. Data on primate PGC specification remain sparse. Existing data suggest that human PGCs may emerge in the nascent AM prior to the gastrulation. Human PGC specification requires additional studies for clarification. For peri-implantation mouse and human embryos, only their embryonic regions are shown.