Figure 3. Proton titration and pH-dependent properties of the catalytic dyad residues in renin.
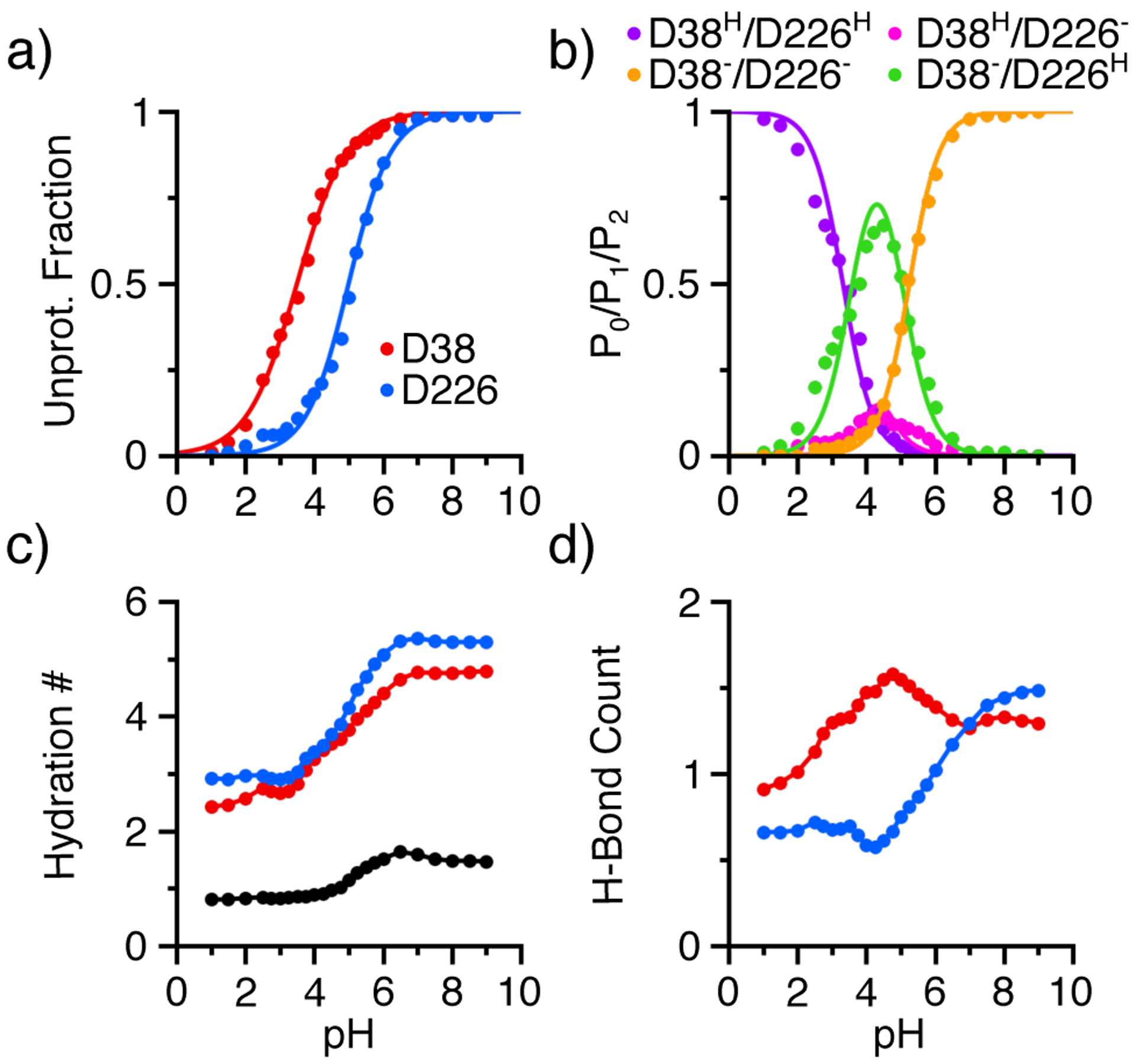
a) Unprotonated fractions of Asp38 (red) and Asp226 (blue) as a function of pH. The lines are the best fits to the generalized Henderson-Hasselbalch equation. b) Probabilities of the doubly deprotonated (P0, orange), singly protonated (P1, magenta and green), and doubly protonated (P2, purple) states. c) Hydration number of Asp38 (red) and Asp 226 (blue) as well as bridge water (black) between the catalytic dyad residues as a function of pH. d) Total number of hydrogen bonds formed by Asp38 (red) and Asp226 (blue) as a function of pH. The number of hydrogen bonds is calculated as the sum of the occupancies of individual hydrogen bonds (see Figure 4). Data from the simulation run 1 was used.
