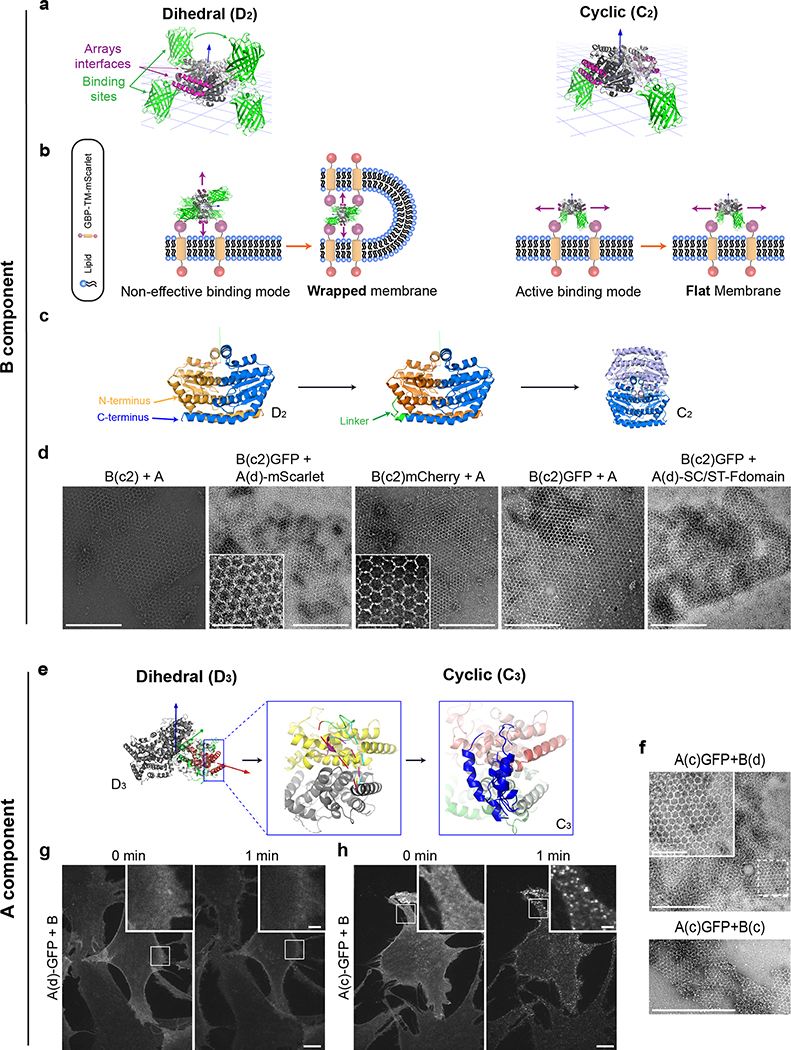
Extended Data Figure 8. Component desymmetrization.
(a-d) B-component desymmetrization. (a) left panel: model of the B component dihedral homooligomer (gray, with the arrays forming interfaces in purple) with GFP fusions (green), blue arrow pointing towards a perpendicular direction to the plane. Right panel: model of a cyclic B component with only two GFP fusions both facing to one vertical direction, note the purple region remain unchanged. (b) Left panel: illustration of the consequences of the binding of a dihedral homooligomer to a flat surface like a lipid bilayer through GFP/GBP interactions: array interfaces are either blocked or facing a direction which is not parallel to the plane. This thereby may induce membrane wrapping and assembly block because propagation interfaces are facing the membrane. Right panel: Ideal binding conformation with the purple arrows indicating the propagation direction when a cyclic component binds to the same membrane. This does not induce any membrane remodelling. (c) schematics of the linker insertion protocol. In the D2 dimer, C- and N-terminal ends are adjacent (left panel, arrows pointing on the terminals). A linker is designed to connect the two (middle panel) resulting in approximately twice as big a monomer which forms a C2 homooligomer (right panel). (d) negative stain EM images of arrays made of B(c) or B(c)gfp and various A components. (e-h) A component desymmetrization. (e) Left panel: A component dihedral (D3) model, two monomers (colored green to red) and red arrow pointing on the designed array interface direction. Middle panel: Various fragments build between the C-term of one monomer to different positions near the N-term of the second monomer. Right panel: Model of the cyclic A components with the new linkers indicated in blue, note that again arrays interfaces remain unchanged. (f) negative stain EM screening for hexagonal assemblies. Top panel shows cyclic A components genetically fused to GFP (A(c)gfp) with dihedral B components, while in the bottom panel both components are cyclic. (g-h). Cyclisation of the A component enables array assembly on cells. Stable NIH/3T3 cells constitutively expressing GBP-TM-mScarlet were incubated with 1μM A(d)gfp (g) or 1μM A(c)gfp (h), rinsed in PBS, then 1μM unlabelled B was added and cells were imaged by spinning disk confocal microscopy. Images correspond to a single confocal plane of the GFP channel. On the contrary to dihedral A, cyclic A enables rapid array assembly on cells, as seen by the characteristic appearance of diffraction limited, GFP-positive spots (see inserts and also Fig. 4 and main text). See also figure S7 in the supplemental material for additional discussion, rationale of component desymmetrization, and computational protocol. Scales Bars: (d) 500 nm (100 nm in inserts); (g,h) 10 μm, 2 μm for insets.