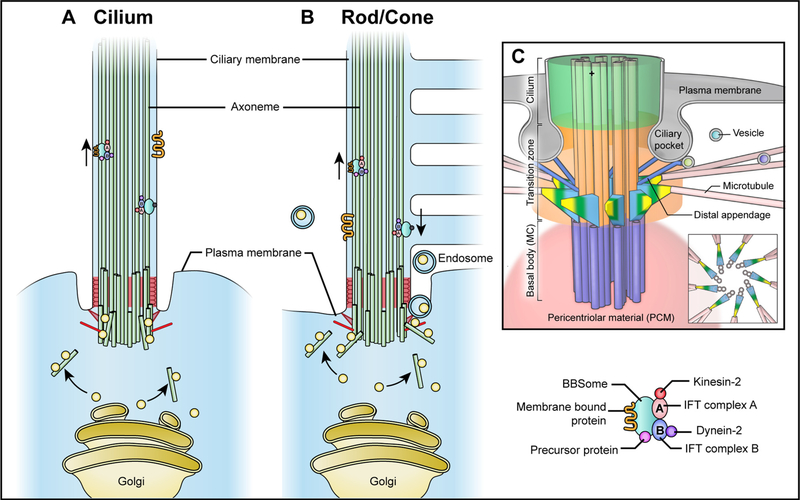
Fig. 3.
Intraflagellar transport (IFT) of the general primary cilium (A) and photoreceptor outer segments (B). A schematic of the base of primary cilium is shown at (C). The IFT machineries are composed of the microtubule motors (kinesins and dyneins), IFT complex (A and B) and accessory proteins (e.g. TULP3, the BBSome). Most ciliary proteins are trafficked to the base of the primary cilia from the post-Golgi network through microtubules in vesicles with the accessory proteins (green oval), which serve as membrane adaptors for specific cargo proteins and IFT complexes. Complex A (pink oval) and complex B (blue oval) move along the microtubule together, yet they have distinct biochemical constituents and functions. Complex B interacts with plus end-directed kinesin-2 motors (red ball) and participates in anterograde transport from the ciliary base to the tip, which is essential for cilia assembly and maintenance. Complex A binds to a minus end-directed motor cytoplasmic dynein-2 (purple ball), which is responsible for retrograde IFT to move cargo proteins from the ciliary tip to the base. In photoreceptors with constant and rapid renewal of the outer segment, besides the conventional pathway by IFT machineries, highly enriched phototransduction proteins (e.g. rhodopsin) can also be transported through recycling endosomes.