Figure 1.
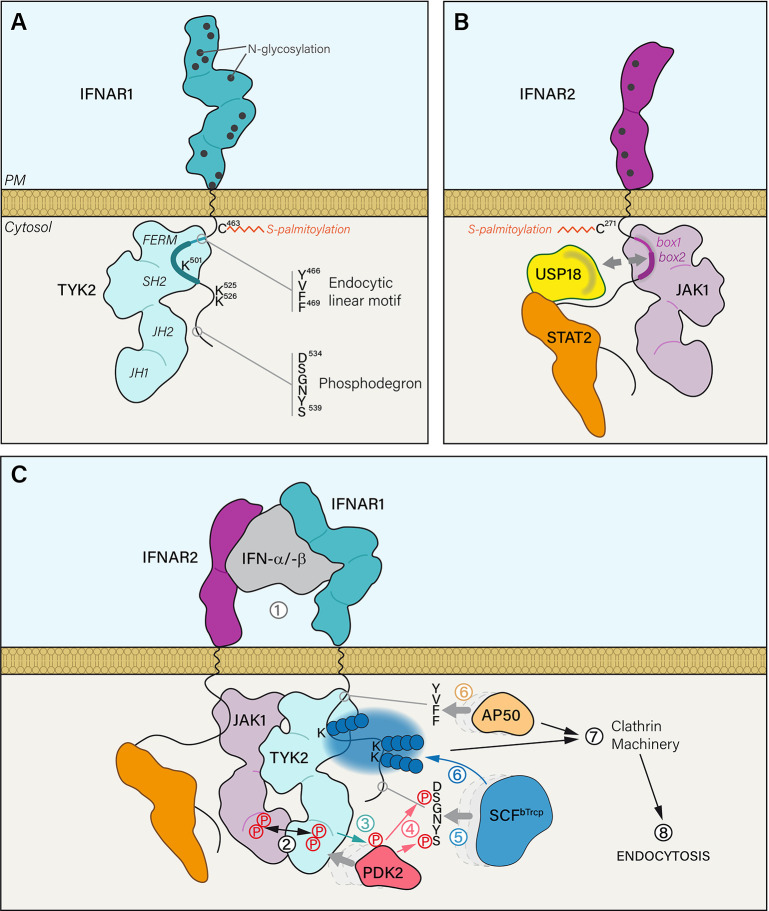
(A) The extracellular part of IFNAR1 is composed of four domains and exhibits 12 residues that are potentially N-glycosylated. IFNAR1 is S-palmitoylated on the PM proximal Cys463 residue. IFNAR1 cytosolic tail interacts with TYK2 kinase FERM and SH2 domains through a minimal region that corresponds to 486–511 residues (thick blue line). IFNAR1 interaction domain with TYK2 can be extended to a maximal region from residues 465 to 511 (thick and thin blue line) that also covers a canonical tyrosine-based linear endocytic motif 466YVFF469. IFNAR1 cytosolic tail has three lysine residues that can be ubiquitinated and a phosphodegron motif. (B) The extracellular part of IFNAR2 is composed of two domains presenting five putative N-glycosylation sites. IFNAR2 is meant to be S-palmitoylated on two Cys residues: one near the PM (Cys271) and another one less likely, closer to the C-terminal part (Cys395). IFNAR2 interacts with its associated JAK1 kinase through cytosolic tail box 1 and box 2 domains. At steady state, ubiquitin specific peptidase 18 (USP18) interacts with the DNA-binding and coiled-coil domains of STAT2 but also with IFNAR2 box 1 and box 2 domains. Therefore, it can compete with JAK1 binding on IFNAR2. (C) (1) IFN-α/-β binding to IFNAR1 and IFNAR2 subunits triggers several mechanistic events leading to the internalization of the receptor complex. (2) IFNAR associated JAK kinases are brought in close proximity resulting in the concomitant tyrosine phosphorylation of JAK1 and TYK2 on Tyr1022-Tyr1023 and Tyr1054-Tyr1055 residues, respectively. (3) Activated TYK2 can then phosphorylate the serine/threonine kinase PDK2 which in turn (4) phosphorylates the two serine residues of the IFNAR1 phosphodegron 534DSGNYS 539. It acts as a docking site (5) for the Skp1-Cullin1-F-box complex E3 ubiquitin ligase (SCFβTrcp). (6) Once bound to IFNAR1, SCFβTrcp is able to polyubiquitinate (blue spheres) lysine residues 501, 525 and 526 by adding Lys48 and Lys63 linkages. In parallel, the endocytic linear motif 466YVFF469 recruits AP50, the μ2 subunit of AP2 adaptor complex. (7) Together, AP50 binding and IFNAR1 polyubiquitination trigger the association of IFNAR receptor complex with the clathrin machinery and its endocytosis via clathrin-coated vesicles.