Figure 2: Central infusion of TRV027 increases water intake.
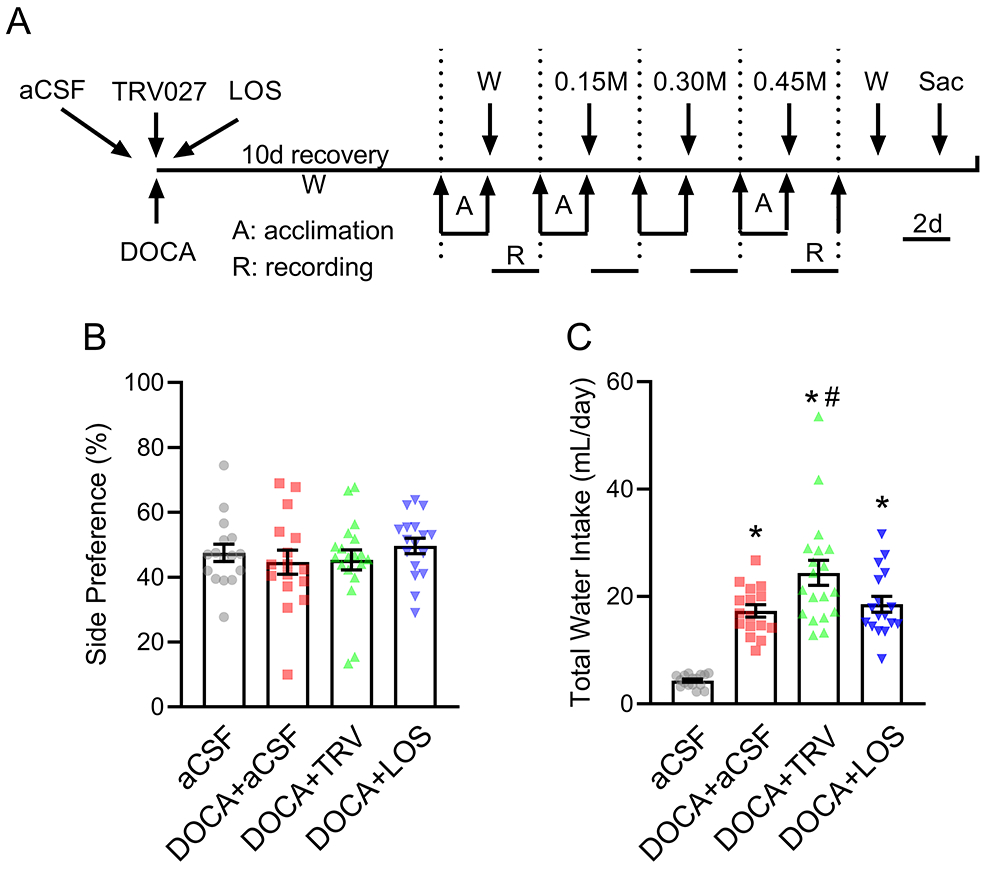
A) Schematic of the experimental protocol to assess drinking behavior. Mice were subjected to subcutaneous implantation of DOCA pellets concomitant with intracerebroventricular (ICV) infusions of either artificial cerebrospinal fluid (aCSF), TRV027, or losartan (LOS). A control group with no DOCA infusion and ICV vehicle infusion was included. Subsequently, mice were subjected to 4 consecutive trials in which mice were presented with a choice between 2 burettes. Each trial consisted of 2 days of acclimation and 2 days of recording. To normalize from the influence of side-bias, the burette positions were interchanged every 24 hours and all drinking data were calculated as the average between 2 days. W: access to water only, 0.15M: access to water vs 0.15M NaCl; 0.30M: access to water vs 0.30M NaCl, 0.45M: access to water vs 0.45M NaCl; Sac: sacrifice. B-C) Side preference (B) and total daily water intake (C) were measured when mice were presented with 2 burettes filled with tap water. Data are expressed as mean±SEM. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons procedure. *p<0.0001 compared with aCSF; #p<0.05 compared with DOCA+aCSF. N values are: aCSF (n=16), DOCA+aCSF (n=16), DOCA+TRV027 (n=19), DOCA+LOS (n=17). The top data point for DOCA + TRV in panel C was a statistical outlier. However, repeating the analysis by excluding the outlier preserves the statistical significance (p=0.03). Thus, the point was retained for transparency.
