Figure 3.
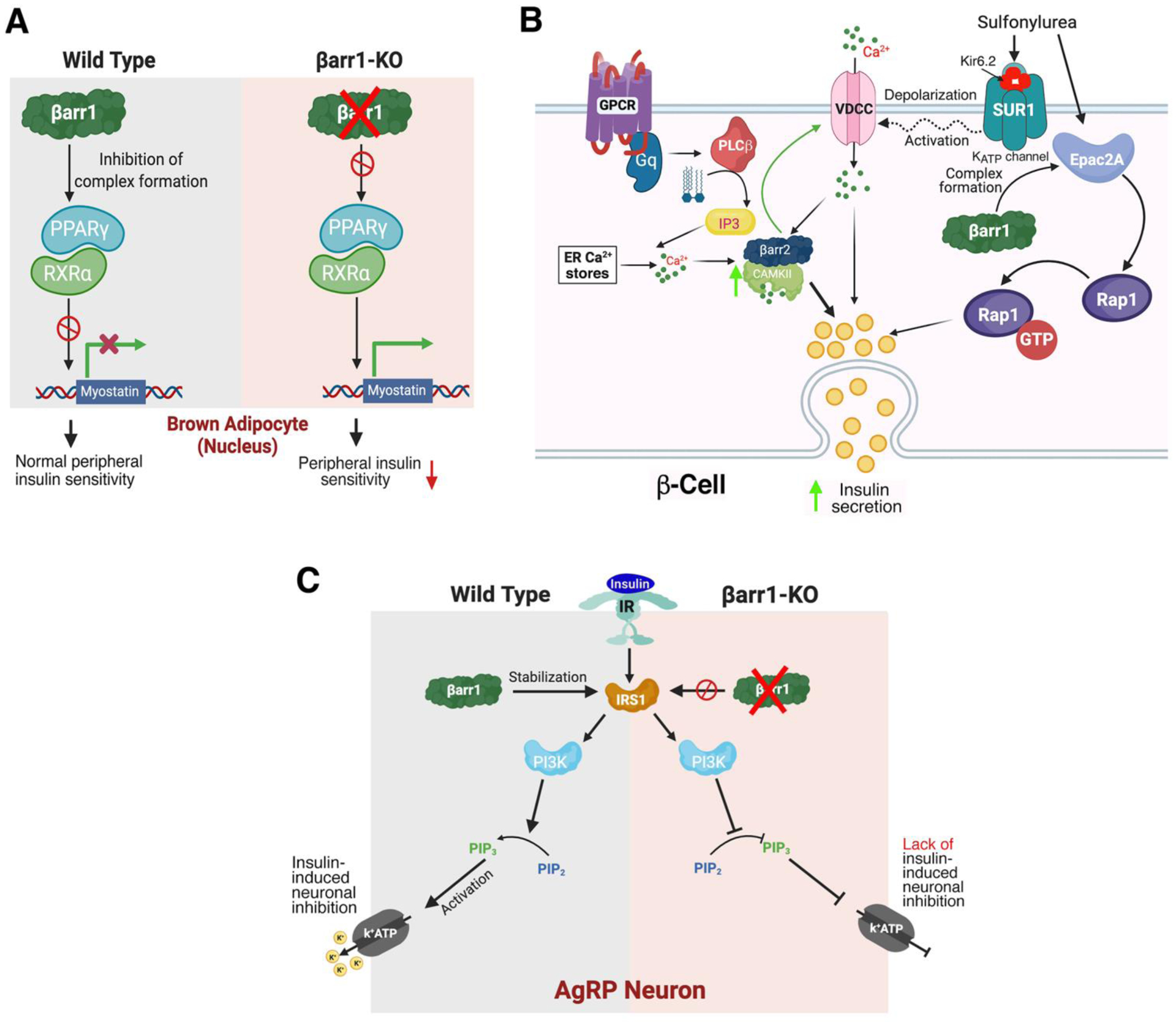
Non-canonical functions of β-arrestins in mouse adipocytes, pancreatic β-cells, and AgRP neurons. A) Adipocytes. In brown adipocytes from wild type mice, βarr1 binding to the PPARγ/RXRα complex is predicted to prevent activation of the myostatin (Mstn) promoter [51] In the absence of βarr1, the PPARγ/RXRα complex can activate the Mstn promoter, leading to increased plasma Mstn levels which cause peripheral insulin resistance [51]. B) β-Cells. The left side of the cartoon shows that βarr2 is required for the proper function of CAMKII in β-cells. βarr2 is predicted to form a complex with CAMKII that stimulates CAMKII activity in β-cells [21]. Activated CAMKII stimulates insulin secretion via phosphorylation of various signaling proteins involved in insulin exocytosis and has been shown to facilitate glucose-dependent calcium influx by acting on VDCCs [89]. Impaired CAMKII activity can fully account for the metabolic impairments observed with beta-βarr2-KO mice [21]. The right side of the cartoon shows that β-cell βarr1 is required for the proper function of most sulphonylurea drugs (SUs). By binding to the SUR1 subunit of the β-cell ATP-gated K+ channel, SU drugs cause channel closure, triggering membrane depolarization, Ca2+ influx through VDCCs, and eventually insulin secretion. Most SUs also stimulate the formation of a βarr1/Epac2a complex, which promotes Rap1-mediated insulin secretion [22]. C) AgRP neurons. In AgRP neurons from wild type mice, insulin induces hyperpolarization and reduced firing frequency by activating a pathway that leads to the opening of KATP channels. In the absence of βarr1, insulin-mediated inhibition of AgRP neurons is abolished, most likely due to reduced stability of IRS1 (IRS1 is stabilized by βarr1 in wild type neurons) [83]. AC, adenylyl cyclase; DAG, diacylglycerol; ER, endoplasmic reticulum; IP3, inositol trisphosphate; IR, insulin receptor; IRS1, insulin receptor substrate 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; PPARγ, peroxisome proliferator-activated receptor γ; RXRα, retinoid X receptor α, VDCC, voltage-dependent calcium channel.
