Abstract
Nitric oxide, NO, has been explored as a therapeutic agent to treat thrombosis. In particular, NO has potential in treating mechanical device-associated thrombosis due to its ability to reduce platelet activation and due to the central role of platelet activation and adhesion in device thrombosis. Nitrite is a unique NO donor that reduces platelet activation in that it’s activity requires the presence of red blood cells whereas NO activity of other NO donors is blunted by red blood cells. Interestingly, we have previously shown that red blood cell mediated inhibition of platelet activation by adenosine diphosophate (ADP) is dramatically enhanced by illumination with far-red light that is likely due to photolysis of red cell surface bound NO congeners.
We now report the effects of nitrite, far-red light, and their combination on several measure of blood coagulation using a variety of agonists. We employed turbidity assays in platelet rich plasma, platelet activation using flow cytometry analysis of a fluorescently labeled antibody to the activated platelet fibrinogen binding site, multiplate impedance-based platelet aggregometry, and assessment of platelet adhesion to collagen coated flow-through microslides. In all cases, the combination of far-red light and nitrite treatment decreased measures of coagulation, but in some cases mono-treatment with nitrite or light alone had no effect. Perhaps most relevant to device thrombosis, we observed that platelet adhesions was inhibited by the combination of nitrite and light treatment while nitrite alone and far-red light alone trended to decrease adhesion, but the results were mixed.
These results support the potential of combined far-red light and nitrite treatment for preventing thrombosis in extra-corporeal or shallow-tissue depth devices where the far-red light can penetrate. Such a combined treatment could be advantageous due to the localized treatment afforded by far-red light illumination with minimal systemic effects. Given the role of thrombosis in COVID 19, application to treatment of patients infected with SARS Cov-2 might also be considered.
Keywords: Nitric Oxide, Thrombosis, Light therapy, Photobiomodulation, clotting, nitrite, red blood cell
1. Introduction
Clotting is a necessary process in hemostasis and as part of wound healing. However, uncontrolled clotting or thrombosis in the absence of a wound can lead to vascular occlusion, ischemia and death. Device thrombosis is a significant complication in otherwise life-saving interventions including hemodialysis catheters, stents, extracorporeal devices (including those used in cardiopulmonary bypass surgery and extracorporeal membrane oxygenation), and in left and right ventricular assist devices [1]. Platelet adhesion, activation, and aggregation play major roles in the initiation and propagation of device thrombosis [1]. Standard care often involves use of systemic anti-coagulants and/or anti-platelet agents but this can lead to bleeding and, in some cases, heparin-induced thrombocytopenia [2; 3]. Clearly, methods to locally reduce thrombosis would be advantageous. One strategy has been to localize release of an antithrombotic drug (such as heparin) to the location of the device by surface modification of the device. However, the clinical efficacy of these approaches has been mixed and not made it so that systemic anticoagulants are unnecessary [1]. Moreover, the drug available in these devices is finite and thus is eventually used up.
The ability of NO to inhibit platelet activation is well-known [4–11]. Use of NO as a treatment of thrombosis has been explored [12]. Others have actually developed nitric oxide (NO) releasing coatings (similar to what is proposed here) but these have suffered from distribution of NO due to its short half-life and challenges in the kinetics of NO-releasing agents [1; 13]. In addition, the ability of NO itself and several NO congers (including S-nitrosoglutathione) to inhibit platelet activity is reduced by the presence of red blood cells (RBCs) which are abundant in blood [14]. This is due to the well-known dioxygenation reaction of NO and oxygenated hemoglobin (Hb)
| (Eq. 1) |
governed by a rate constant of 6–8 × 107 M−1s−1 and where MetHb is methemoglobin [15–17]. On the other hand, the ability of nitrite to effect NO-mediated inhibition of platelet activation requires the presence of RBCs [14; 18]. Indeed, in the presence of partially deoxygenated RBCs, 1 μM nitrite is the most effective NO donor and this concentration is achievable in human plasma through intake of dietary nitrate whereas it is 1000 times more than physiological levels of NO and nitrosothiols [19–21].
Nitrite is known to react with deoxygenated Hb, [22]
| (Eq. 2) |
However, NO produced within the RBC must be rapidly scavenged through the reaction described in Equation 1. Thus, the reaction between Hb and nitrite must be more complex. We have shown RBC surface thiols are necessary for nitrite bioactivation by RBCs and hypothesized that a nitrosothiol or other thiol-bound NO congener forms in the bioactivation process [23]. Regardless of mechanism, it is remarkable that 1 μM nitrite (which forms NO inside the RBC) is as or more effective in inhibiting platelet activation than the same concentrations of NO itself and nitrosothiols when applied externally to RBCs and platelets [14]. We thus propose that nitrite is the best NO-based anti-platelet agent in blood.
Although it may be the best NO-based anti-platelet agent, nitrite monotherapy would still likely be too weak for many applications. However, we recently discovered that five minutes of illumination with far-red light (660 nm, 560 mW) dramatically enhanced inhibition of platelet activation by ADP as measured by a decrease in activated GPIIb/IIIa complex measured by PAC-1 binding [24]. We attributed this phenomenon to photolysis of RBC surface thiol bound NO species as illustrated in Figure 1. Additive or synergistic effects of illumination with nitrite therapy could be advantageous in therapeutic applications aimed at local NO release and inhibition of thrombosis with minor systemic effects. In this paper, we examined effects of light, nitrite and their combination on various measures of platelet activity and clotting using different agonists.
Figure 1.
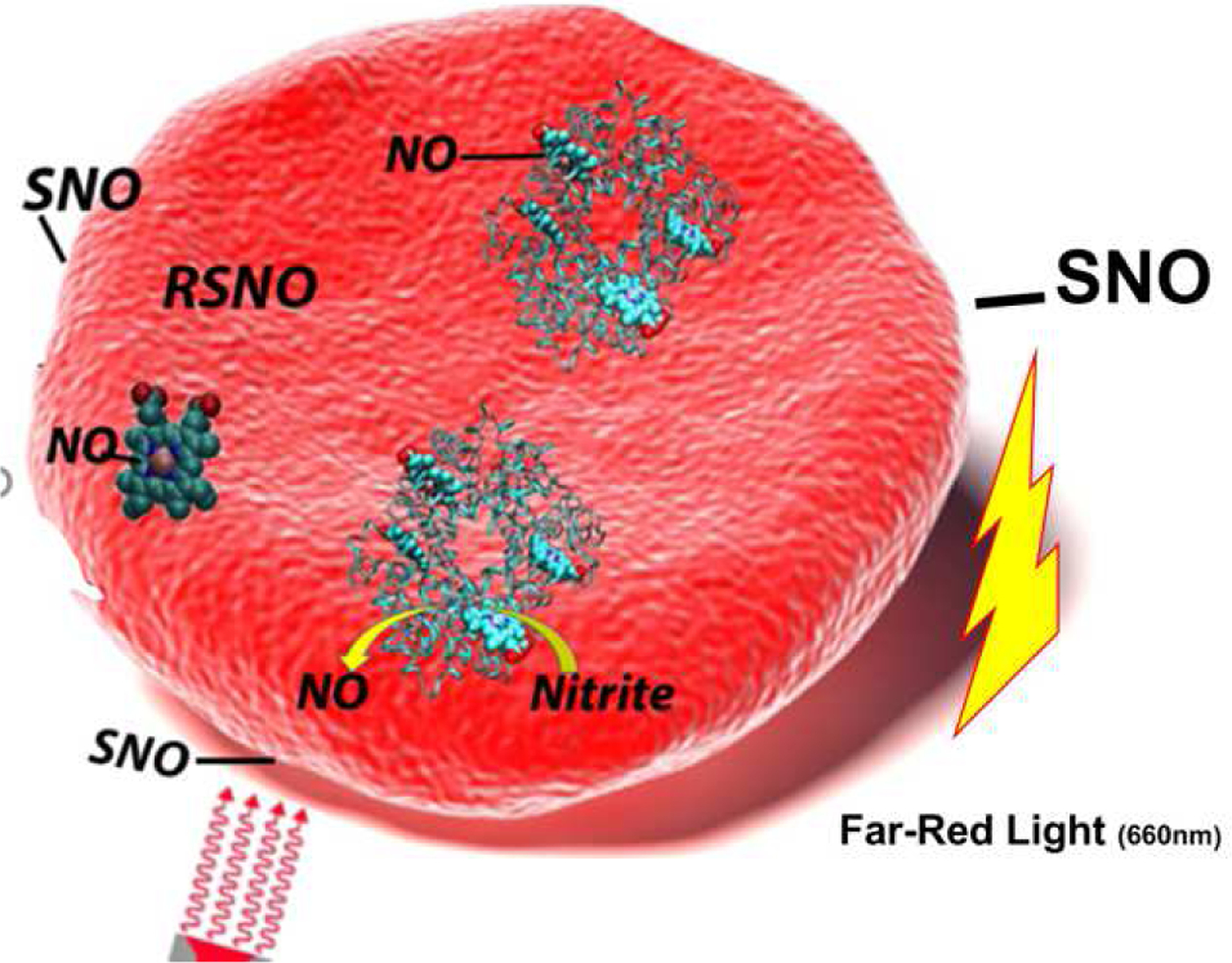
Proposed mechanism of far-red light potentiated NO release from red blood cells. Nitrite reacts with deoxygenated hemoglobin to make NO which can lead to nitrosyl heme and/or nitrosothiol (RSNO) formation. These NO species migrate to the membrane surface forming a photolabile NO cogener/surface thiol bond
2. Materials and Methods
2.1. Materials.
Whole blood was collected by venipuncture from healthy volunteers after obtaining informed consent following a protocol approved by the internal review board at Wake Forest University Health Sciences (IRB##BG02–168). Pac-1 and Per-CP CD 61 antibodies were purchased from BD Biosciences, μ-Y-Shaped flow channel slides were purchased from ibidi, Rat tail Collagen I was from Hart Biologicals. All other materials were obtained from Sigma Aldrich except as noted otherwise.
2.2. Far-red light treatment.
A “deep red” LED source (M660L4, Thorlabs, NJ) at a center wavelength of 660 nm was used to illuminate the samples through an aspheric condenser lens (ACL5040U-B, Thorlabs, NJ) and a focusing lens (LA1050-B, Thorlabs, NJ). The setup produced a 1 cm diameter spot on the sample. The incident light power on the sample was measured by a power meter (1815-C, Newport, CA) to be 560 mW.
2.3. Platelet Activation.
Preparation of platelets and deoxygenated RBCs were performed as described previously [24]. Platelet and RBC mixtures were incubated at room temperature (21°C) with and without nitrite (10 μM) and with and without far-red light for five minutes followed by addition of a platelet agonist to initiate activation. The mixture was then incubated for another five minutes. FITC labeled PAC-1 and Per-CP labeled CD61 antibodies were added to 10 μL of the sample for an additional 15 minutes before fixation with 1% buffered formalin. Platelet activation was quantified as percent PAC-1 positive using a BD Bioscience FACS Calibur flow cytometer.
2.4. Platelet aggregation.
The effect of nitrite, collagen and far-red light on whole blood was determined by the impedance aggregometry using Multiplate® platelet function analyzer from diaPharma (West Chester, OH) at the Wake Forest University School of Medicine. Multiplate® analysis takes place in a single-use test cell which incorporates dual sensors and a Teflon-coated stirring magnet. Blood was drawn in citrate anticoagulant tubes and degassed for thirty minutes under nitrogen without bubbling. Whole blood (400 μl) was diluted in degassed saline and hematocrit was adjusted to 17.5%, incubated with and without 20 μM nitrite, and exposed to far-red light for 5 minutes (as described elsewhere in the method section) at room temperature. After 5 minutes of incubation, 300 μl of reaction mix was added into the micro cell of the multiplate platelet function analyzer. The aggregation was induced by 2.5 μg/ml collagen in presence of 0.9% NaCl and 1.5 mM CaCl2 according to the manufacturer’s recommendation. Aggregation was induced for 5 minutes at 37° C and platelet aggregation was recorded by the automated system. The system calculated the area under the aggregation curve to quantify aggregation in terms of aggregation units (AU). Statistical analysis and significance were determined by GraphPad Prism software v 5.
2.5. Turbidity measurements of clotting.
A microplate reader, which uses spectrophotometry to measure the optical density of formed clots at a certain wavelength, was employed for lag time and clot 50% lysis measurements [25]. Citrated whole blood samples were spun down (300 g for 7 minutes) for collecting platelet rich plasma (PRP). Four samples with a final hematocrit of 20% using washed RBCs with and without nitrite treatment, and with and without far-red light treatment were prepared with plasma mixtures and activation mixtures as previously described.24 The plasma mixture contained plasma (720 μL), 480 μL of 0.05M phospholipid (Rossix, AB, Sweden), 60 μL of 25 ng/ml tPA, and 420 μL of assay buffer (50mM Tris-HCl, 140mM NaCl, 1 mg/ml BSA, pH 7.4). The activation mixture contained 200 μL assay buffer, 300 μL 250 mM CaCl2, and 200 μL of 12.5 units/ml human alpha thrombin. A final concentration of 10 μM deoxygenated nitrite was added to two sets of both plasma and activation mixtures, while the other two sets were loaded with the same volume of deoxygenated assay buffer. 1 mL of each plasma and activation mixture (both with and without added nitrite) were transferred into four 1-cm path length glass cuvettes (GL14-S, Starnacells) and then were exposed to far-red light. Samples were placed back to back at the focal point and switched places after 5 minutes of exposure.
After the total 10-minute light treatment, the supernatant of all eight degassed samples were collected (300 g for 15 minutes). 100 μL assay buffer, 80 μL of the plasma mixtures supernatant, and 50 μL of the activation mixtures supernatant were injected in three columns of a 96-well microplate, respectively. Using a multichannel pipet, 20 μL of the activation mixture was added simultaneously to the microplate wells containing different plasma mixtures. Clot formation was initiated immediately after injecting the activation mixtures. The plate reader recorded the optical density at 37°C and at 405 nm (or 450 nm depending on the initial turbidity of the supernatants) every 6–8 seconds for 3 hours. The period of time from the start of recording until the clot starts to appear, defined by when the optical density value is 0.015 greater than the clear baseline, is called lag time. The 50% lysis time is defined as when the optical density reaches half its maximum and when it reaches the same point again
2.6. Platelet Adhesion Assay.
A schematic of the apparatus is shown in Figure 2. Partially deoxygenated blood was diluted 1:1 with deoxygenated saline in 50 mL tubes and deoxygenated for an additional 15 to 20 minutes. PBS (10 mL) was flowed through the collagen coated μ-Silde y-shaped from ibidi for 10 minutes. Blood was then loaded into 10 mL syringes with nitrite containing samples having a final concentration of 100 μM. The LED was placed 15 cm from the illuminated sample. The flow-rate on the Harvard pump was set to 500 μL/min and lasted 20 minutes. Slides were then washed for at least 20 minutes with PBS before staining with FITC Pac-1 and Per-CP CD 61 and fixated with 4% buffered formalin. Platelet adhesion was measured using a LSM 710 laser scanning confocal microscope and analyzed using ImageJ for mean fluorescence intensity and the number of adherent particles.
Figure 2.
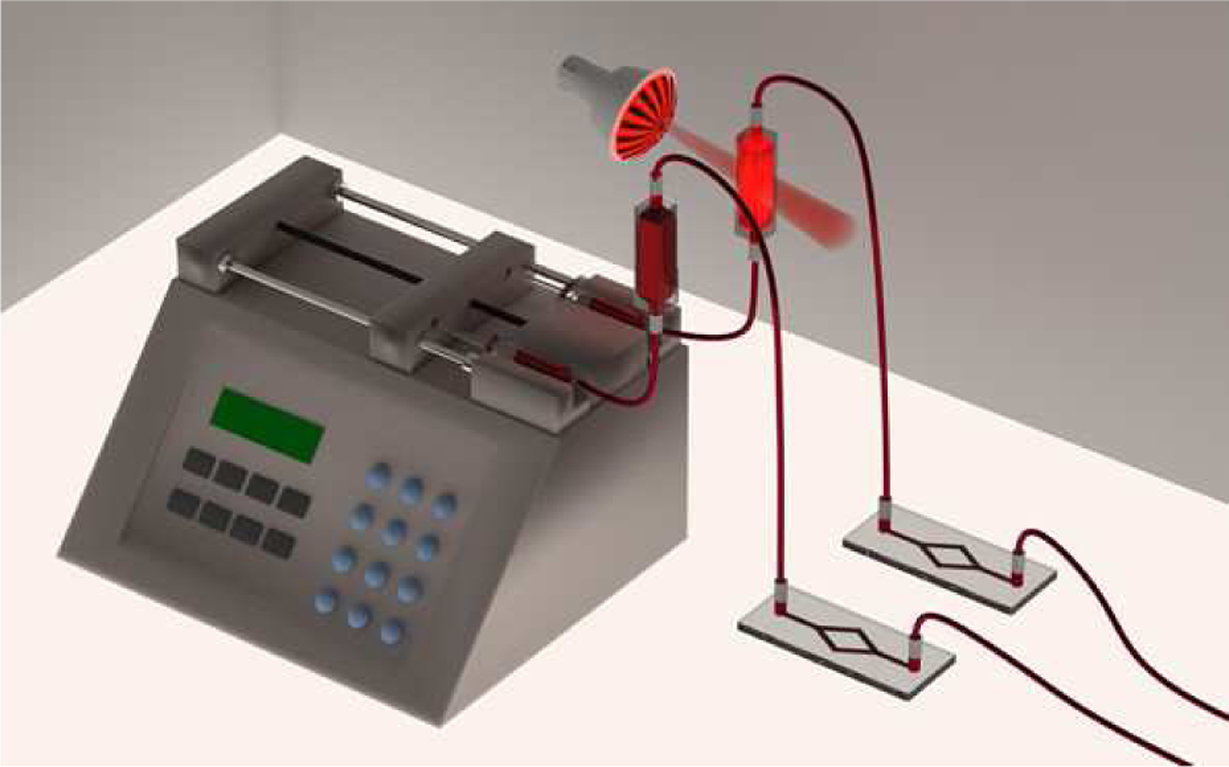
Schematic of flow channel adhesion apparatus. Two 10 mL syringes containing 100 μM nitrite were placed in a Harvard pump with a flow rate of 500 μL/min for 20 minutes. 1-cm path length glass cuvettes were connected to the slides separately. The illuminated sample was located 15 cm away from the LED light source. Slides were washed with PBS for 20 minutes before FITC Pac-1 and Per-CP CD 61 staining.
2.7. Statistics.
In measurements comparing one condition to another, Student’s t-tests were used either paired, if all sets were conducted on the same samples, or unpaired. In comparing multiple groups together, ANOVA was used. Significance was set at p<0.05.
3. Results
3.1. Clotting Lag Time
Our previous results have shown that nitrite and far-red light increase the lag-time thrombin-induced clotting in platelet poor plasma (PPP) and the combination of these treatments was significantly more effective than a single treatment alone [24]. Incubation of the PPP with partially deoxygenated RBCs was necessary for these actions. There were no observed effects on clot lysis time or maximum optical density [24]. We have now further explored the effects of far-red light and nitrite on clotting; this time including platelets in the assays. Figure 3A shows representative curves of turbidity vs time during clotting and fibrinolysis. Lag times and lysis times were quantified and the averages are shown in Figures 3B and 3C. The combined nitrite and far-red light treatment trended to increase lag time but the result was not significant (n = 5, P = 0.11). The lysis time was significantly lower than control for all treatments (light, nitrite, and the combination; n = 5 P < 0.05)
Figure 3.
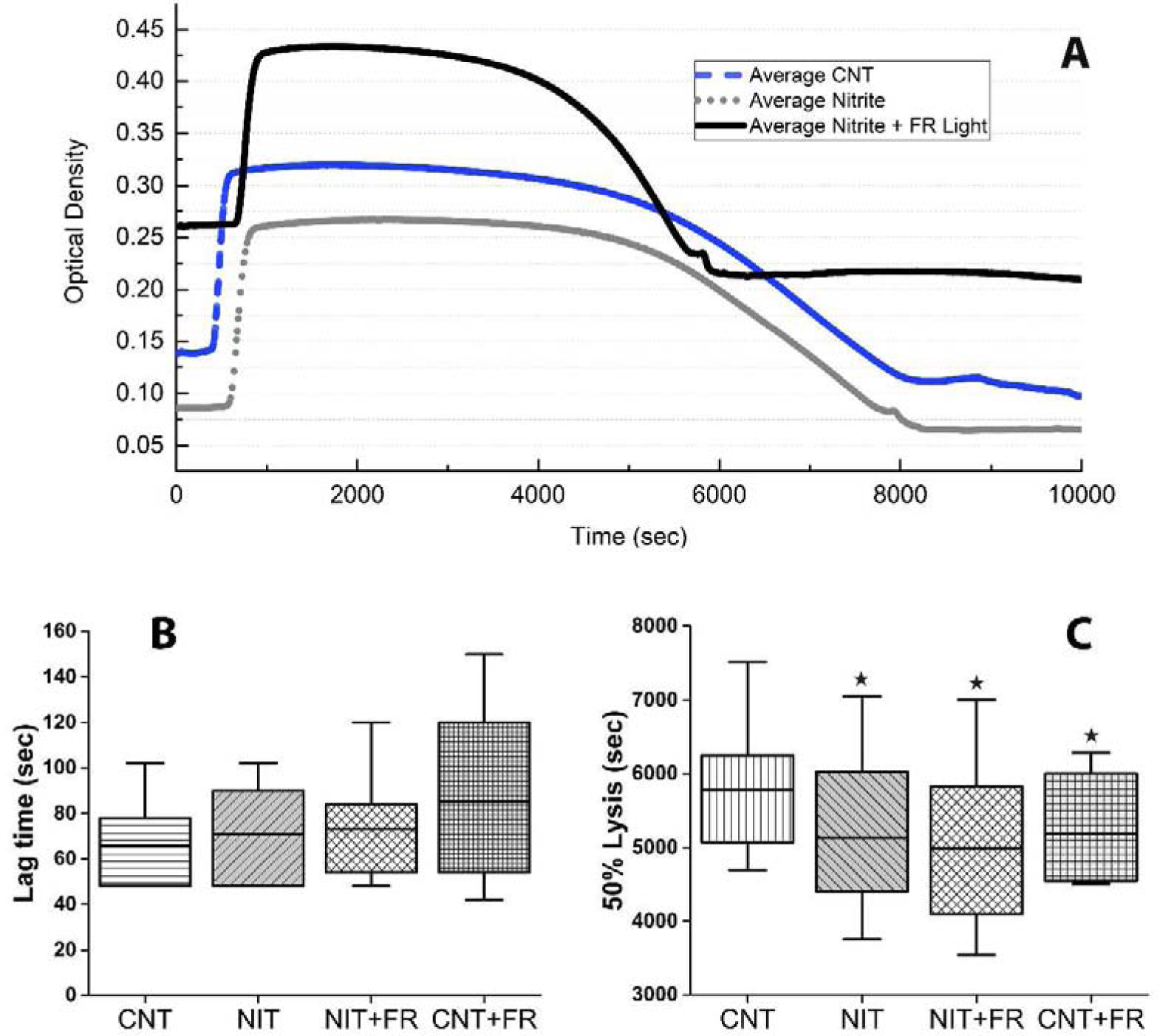
Effects of far-red light and nitrite on clotting in platelet rich plasma. (A) Representative curves for the platelet-rich plasma (PRP) turbidity assay, where control (Cnt) was plotted against nitrite, and nitrite combined with far-red (FR) light treatment. Longer lag time and shorter lysis time is plotted for nitrite+light, nitrite, and Cnt samples respectively. (B) Compared average (n =5) lag times in platelet-rich samples. In the presence of RBCs and platelets, the increasing trend of lag time in 5 minutes of light treatment alone and also combined with nitrite treatment showed longer lag times. The box plots are plotted to show the minimum and maximum values (C) Averaged 50% lysis time for all four conditions. Followed by the significant shorter lysis period for Nit vs the Cnt samples (*P = 0.009, n = 5), Cnt vs Nit + FR Light samples showed the lowest significant lysis (*P = 0.011, n = 5). The substantial averaged 50% lysis time in samples with only light treatments can be seen in this graph as well (*P = 0.038, n = 5). P values here are from paired t-test vs control. The box plots are plotted to show the minimum and maximum values
3.2. Platelt Activation
Employing flow cytometry to measure fluorescently labelled PAC-1 binding to the platelet fibrinogen binding site GPIIAb-IIIA as a measure of platelet activation, we previously showed that light and nitrite inhibit ADP-stimulated platelet activation and the combination of these two treatments has a synergistically enhanced effect [24]. Here we tested the effects of the two treatments in inhibiting platelet activation by other agonists. Figure 4A shows platelet activation using the thromboxane A2 receptor agonist U46619. Baseline activation (before adding U46619) was 1.9 ± 1.5% (data not shown). All data shown in Figure 4 are for experiments conducted in the presence of partially deoxygenated RBCs; there was no difference in pre-treatment activation in the presence and absence of the RBCs (P = 0.7, data not shown). Nitrite effectively inhibited platelet activation by U46619 (n = 6, P < 0.01 paired t-test). Treatment with far-red light in the absence of nitrite trended to reduce platelet activation, but the results were not significant (p = 0.15). The combination of nitrite and far-red light significantly inhibited platelet activation compared to no treatment (p < 0.002) and compared to nitrite treatment alone (p < 0.02). Figure 4B shows platelet activation using thrombin receptor agonist peptide-6 (TRAP). Baseline activation for these experiments (without TRAP) was 3.4 ± 1.8% (data not shown). Nitrite strongly trended to decrease platelet activation by TRAP (p = 0.054). Surprisingly, illumination with far-red light, in the absence of nitrite, actually increased platelet activation by TRAP (P < 0.01, n = 5). The combination of nitrite and far-red light significantly inhibited platelet activation compared to no treatment (P 0.03).
Figure 4.
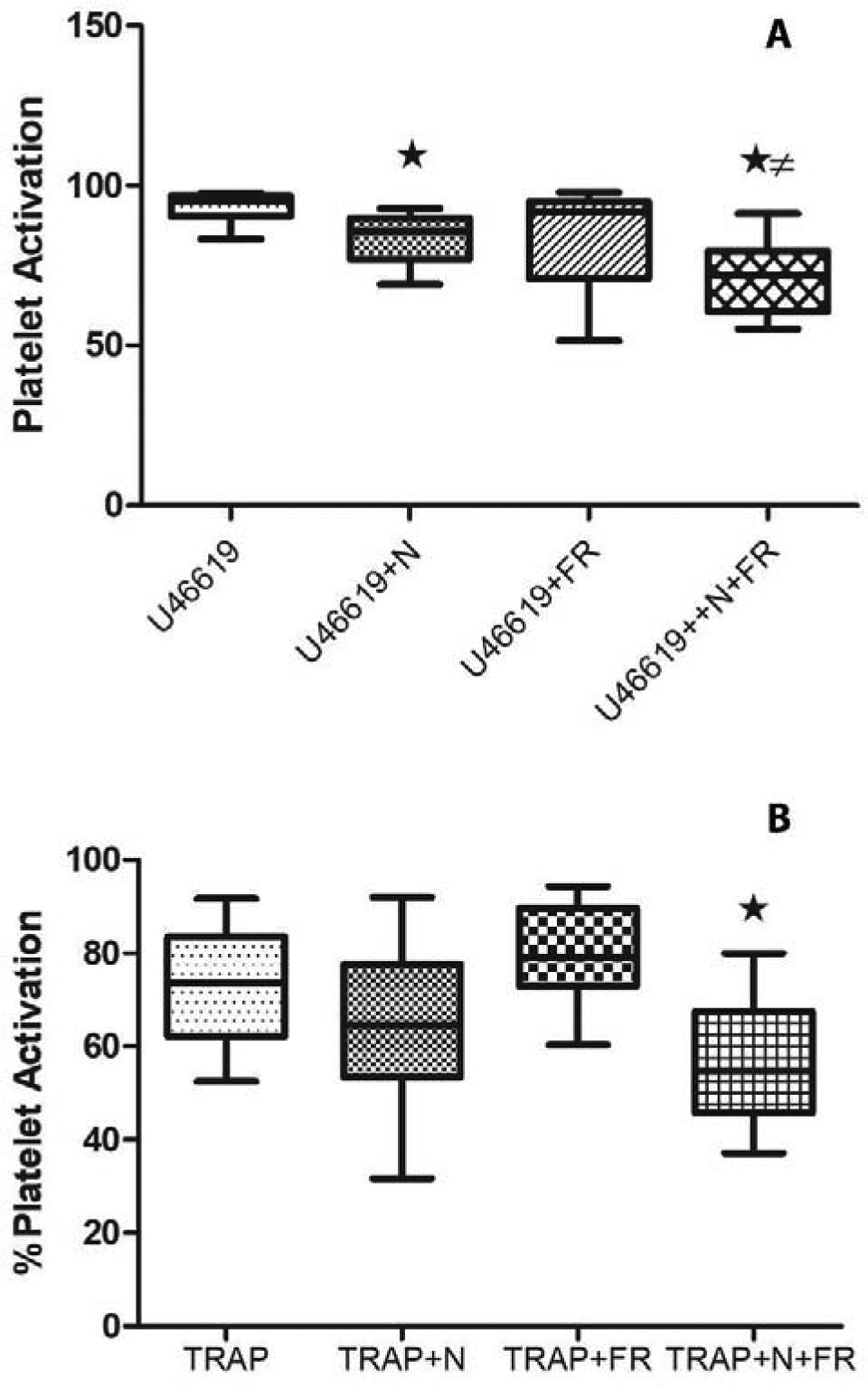
Effects of far-red light and nitrite on platelet activation. Platelet activation is shown for samples containing partially deoxygenated red blood cells in the presence and absence of treatments. The box plots are plotted to show the minimum and maximum values. (A) Platelet activation by thromboxane A2 receptor agonist U46619. In the absence of treatments, average platelet activation was 93.5%. Addition of nitrite (N) significantly reduced activation to 83.7% (n = 6, *P<0.03). Treatment with far-red light (FR) trended to decrease action to 83.3%, but the result was not significant. The combined treatment resulted in average activation of 71%; significantly lower than activation without treatment (n = 6, *P<0.03) and lower than that with nitrite only treatment (n = 6, ≠P<0.03). (B) Platelet activation by thrombin receptor agonist peptide-6 (TRAP). Nitrite trended to reduce activation from 73% to 64.7% (n = 5, P = 0.054) and far-red light treatment actually increased platelet activation to 79.1% (n = 5, P < 0.01). The combined treatment reduced platelet activation to 56.2% (n = 5, *P< 0.03).
3.3. Platelet Aggregation
To further test the effects of nitrite and light on different aspects of clotting, we examined effects on platelet aggregation using an impedance-based Multiplate aggregometer. All experiments were performed in diluted whole blood (17% hematocrit) with collagen as the platelet agonist. Figure 5 panels A-D show representative kinetic profiles of aggregation for nitrite, light treatments along with the control and combined treatment. Figure 5E shows the average amount of adhesion for six separate trials. Nitrite treatment alone significantly reduced platelet aggregation but light treatment had no additional inhibitive effects.
Figure 5.
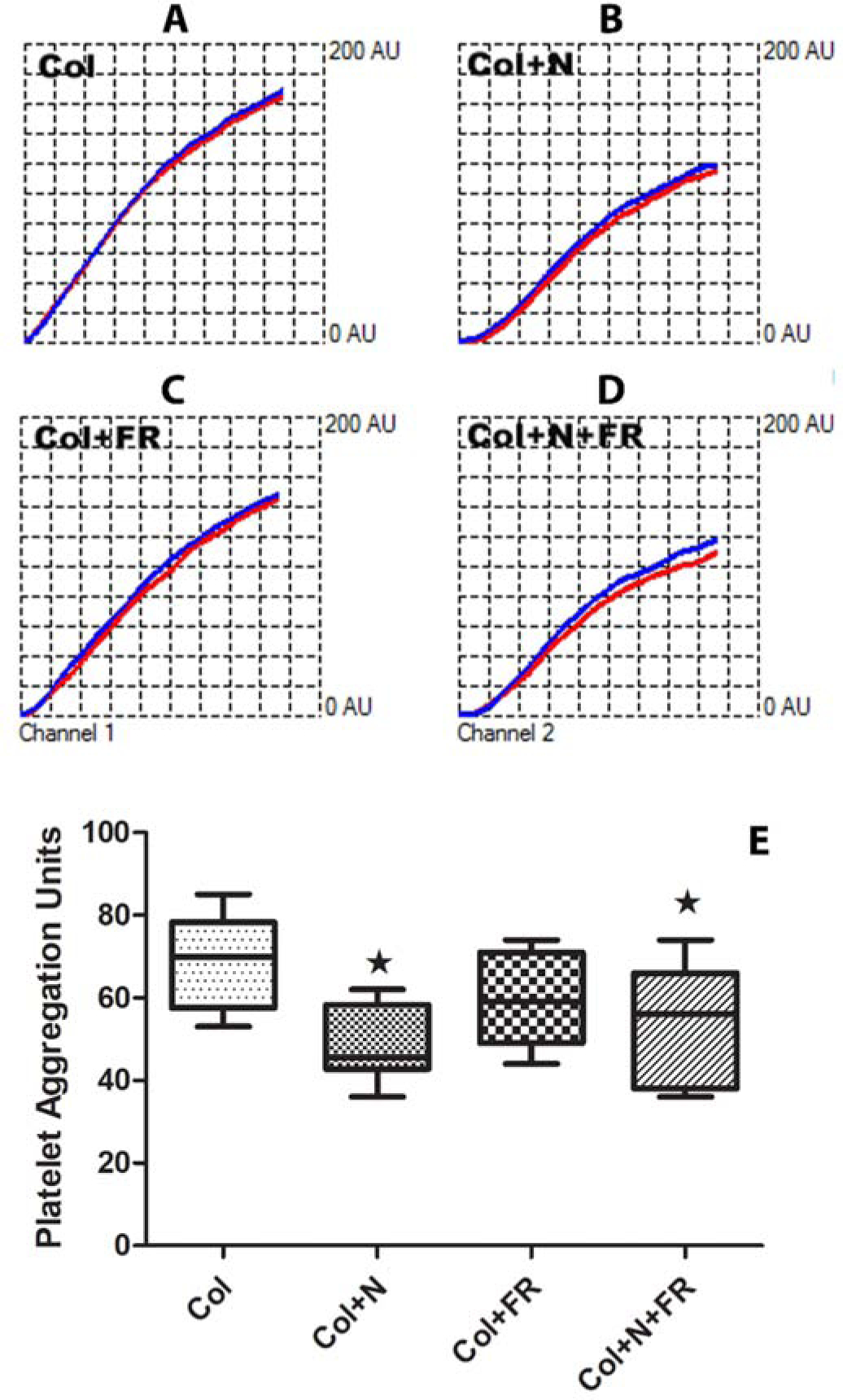
Effects of far-red light and nitrite on platelet aggregation. Aggregation was induced with collagen (Col) in whole blood diluted to 17.5% and monitored using impedance in a Multiplate aggregometer. (A-D) Representative kinetic profiles of aggregation in arbitrary units in the absence of treatment (Col), with nitrite added (N), with far-red light treatment (FR), and the combination treatments. Red and blue traces are from two different electrodes and are expected to be similar to each other. (E) Average results from six experiments. Both nitrite and the combination treatment significantly decreased aggregation compared to no treatment (*P < 0.001), but the combined treatment was not significantly more effective than nitrite alone. Box plots are plotted to show the minimum and maximum values.
3.4. Platelet adhesion
There is an important interplay between platelet activation and adhesion that can lead to pathological consequences in many scenarios including device thrombosis. We examined the effects of nitrite and far-red light treatments on platelet adhesion in collagen coated micro-channels. As illustrated in Figure 2 showing a schematic of the set-up, experiments were performed pairwise, treatment vs control, since we could only flow two samples through the micro-channels at the same speed and conditions at a time. Figure 6 shows representative images for control samples vs those treated with nitrite and far-red light. The platelets were labelled to fluoresce red light for all platelets and green light for activated platelets. The nitrite and light treatments clearly reduce platelet adhesion. The data were quantified using two methods - calculating the mean fluorescent intensity (MFI) and the number of adherent particles. The data from 4–6 paired experiments where 4–6 regions of interest were analyzed in each experiment are shown in Figure 7. All treatments lowered adhesion although the effect was significant only with the combined light/nitrite treatment as measured by both MFI and number of adherent particles while the nitrite treatment alone had significant effect measured by the number of adherent particles but not by MFI.
Figure 6.
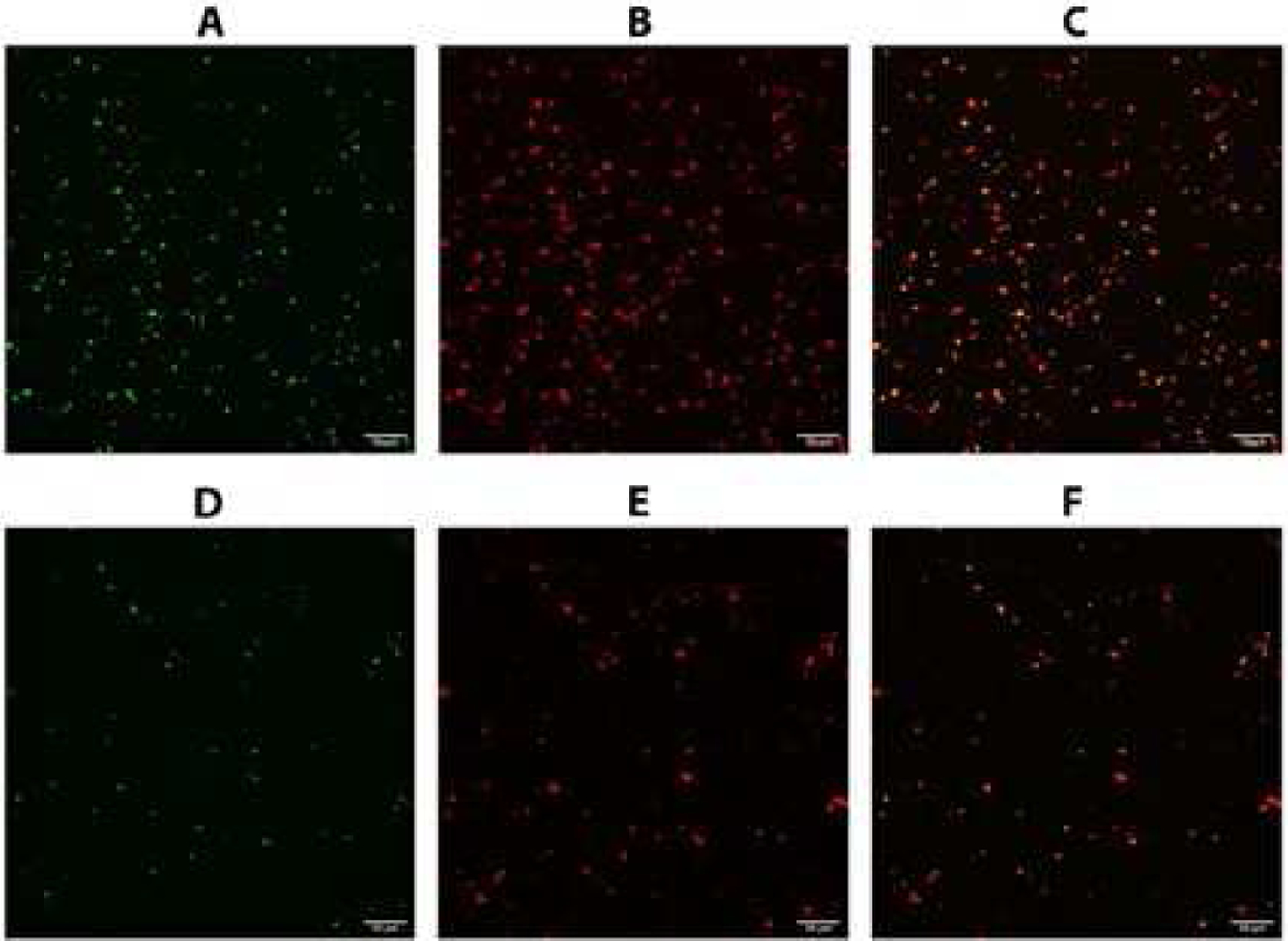
Platelet adhesion under flow conditions. Deoxygenated blood was diluted 1:1 with saline and pumped through collagen-coated microchannels with and without treatments (nitrite and far-red light) as depicted in Figure 2. Adherent platelets were observed using confocal microscopy. Green fluorescence was from activated platelets (FITC-Pac1, A,D) and red fluorescence was for all platelets (Per-CP CD 61, B,E). Merged images are shown in Panels C and F. Top row, A-C, is for samples treated with nitrite and far-red light. Bottom row, D-F, is for control samples. Bar is 50 μm.
Figure 7.
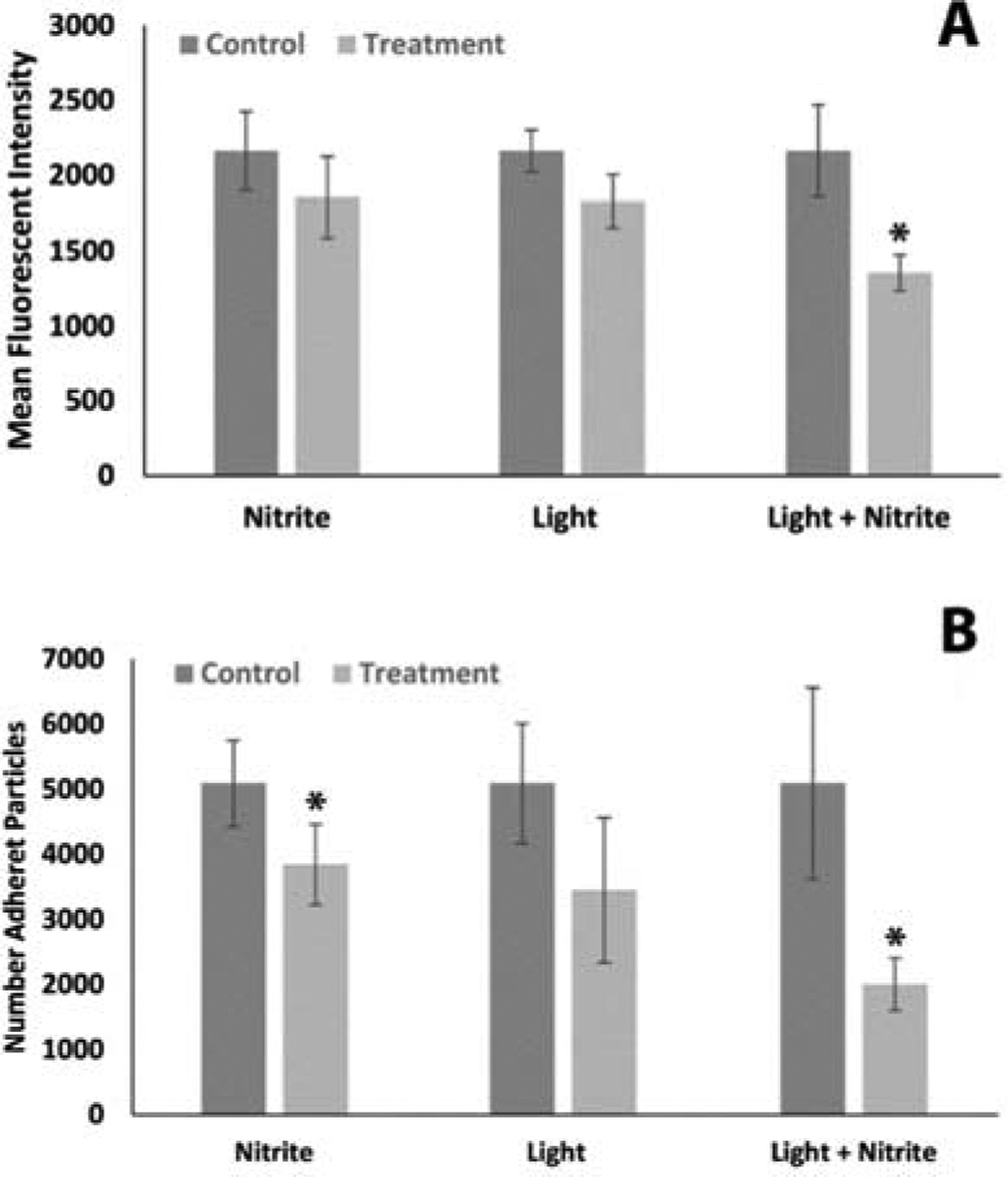
Platelet adhesion averages. Each experiment was run pairwise with one treatment (either nitrite (n = 5), light (n= 4) or the combination (n = 6)) vs control. All data were normalized by the average of all control results divided by the control for each treatment. Each experiment analyzed four different pre-determined regions of interest by mean fluorescent intensity (A) or number of adherent particles (B). *P < 0.05
4. Discussion
Clotting is a complicated process that can be initiated, propagated, and suppressed by several different interactive pathways and agents. We have previously shown that nitrite, and far-red light treatment and their combination increases the lag time in clotting in platelet-poor plasma initiated by thrombin and decreases platelet activation initiated by ADP [24]. In the case of platelet activation initiated by ADP, the combination of nitrite and far-red light dramatically inhibited activation compared to either treatment alone [24]. We have now examined various additional measures of clotting employing several agonists and found that whereas nitrite and far-red light have mixed results in terms of inhibiting clotting, the combination is consistently effective.
Our results examining the kinetics of clotting and fibrinolysis in PRP are different from what we have previously observed in PPP [24]. We had previously observed that the combination of nitrite and light increases lag time but had no effect in fibrinolysis. Here, in PRP, we see a trend in increasing lag time and a significant reduction in the time for fibrinolysis. Although platelets are thought mainly to inhibit fibrinolysis due to clot retraction, their effects are now thought to be more complex [26]. In any case, our treatments may modulate the role of platelets in fibrinolysis which was not observed in PPP.
Conflicting results on platelet measures following monotherapy may be attributable to complex action of NO on platelet activation [27–32]. Although it is widely accepted that NO has anti-platelet effects, several reports have shown evidence for a dual effect of NO on platelet activation where low concentrations of NO are actually stimulatory and higher concentrations inhibitory [28]. In addition, binding of several platelet agonists have been shown to increase NO through platelet nitric oxide synthase which increases cyclic guanosine monophosphate (cGMP) and this leads to activation and increased sensitivity to these agonists [28; 31]. Adding to the complexity, several reports suggest that NO effects in platelets are variable [29] and can act through cGMP-independent pathways,[30] but it should be noted that some of these complexities have been challenged [33]. With these considerations, we propose that light treatment alone may release less NO than nitrite treatment alone or its combination and in some cases have no effect or even (in the case of TRAP) a small stimulatory effect, while the combination treatment consistently releases enough NO to be inhibitory. These notions are presented in Figure 8.
Figure 8.
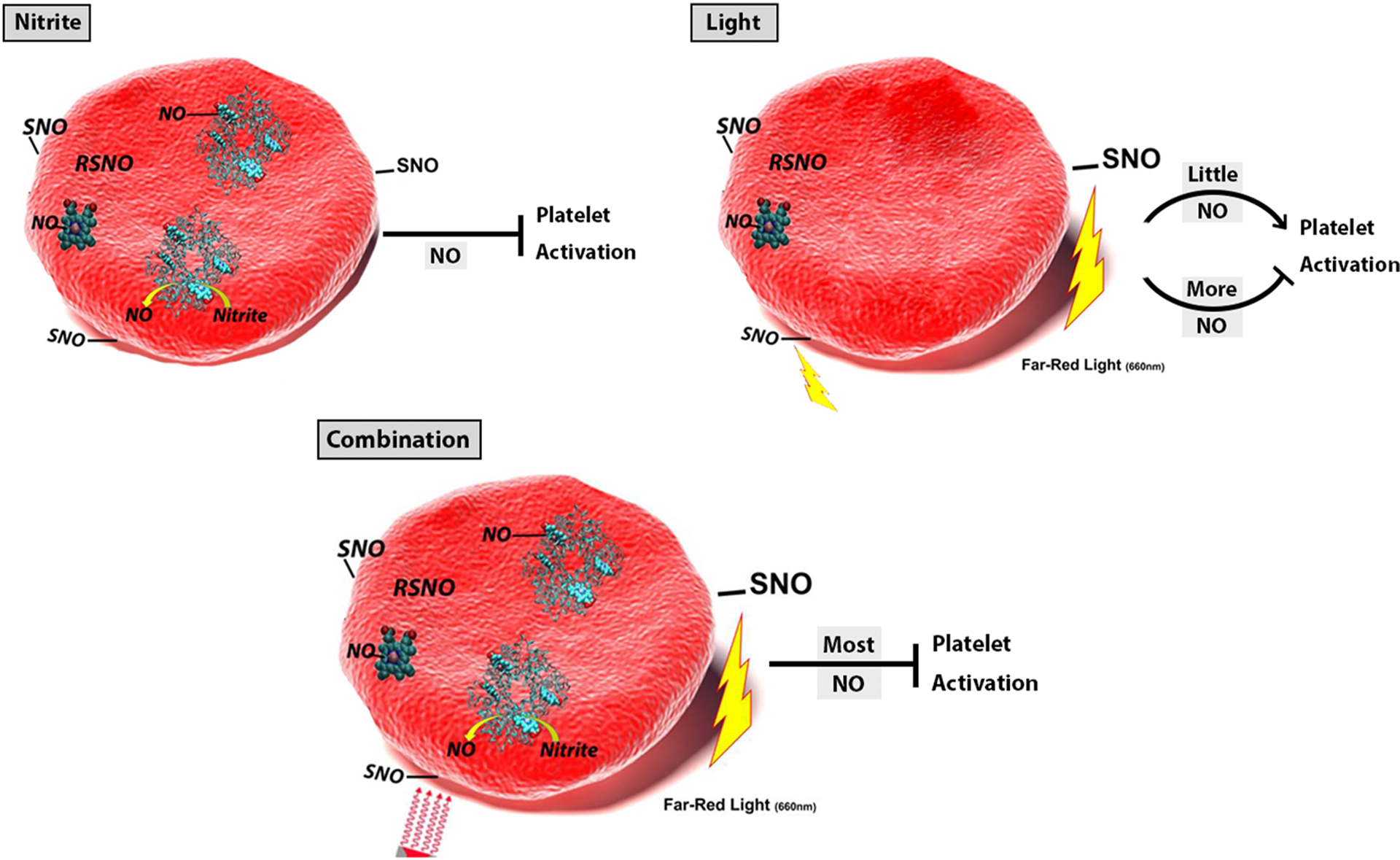
Revised Hypothesis Figure. The effect of treatments on platelet activity depend on the amount of NO made. In the case of nitrite alone, sufficient NO is made so that platelet activation is inhibited. When light alone is applied, the amount of NO made will depend on how much is already bound to the RBCs from previous nitrite bioactivation. When this amount is small, platelet activation is not inhibited and may even be stimulated. For the cases where the nitrite and light is combined, NO formation is maximal and platelet activation is inhibited.
We examined the effects of U46619 and TRAP on platelet activation. Nitrite significantly inhibited platelet activation by U46619 but not TRAP. Far-red light treatment alone had no significant effects on platelet activation. However, the combination treatment significantly decreased platelet activation induced by both U46619 and TRAP. In our earlier work we observed that both nitrite and far-red light inhibited platelet activation by ADP as single treatments and the combination was dramatically more effective than either treatment alone. It is unclear why the treatments did not show identical patterns across the different activating agonists employed. Some of the difference could be due to a difference in efficacy of NO in inhibiting platelet activation by different agonists. Importantly, the only time nitrite treatment alone did not significantly reduce platelet activation (with TRAP) it did trend to do reduce it, supporting NO-mediated inhibition of platelet activation. Light treatment alone trended to decrease platelet activation by U46619 but showed no inhibition using TRAP. As suggested above, these effects by light treatment alone may be attributable to complex affects of NO on platelet activation that includes a stimulatory effect at low levels NO.
Somewhat similar results were obtained when effects of the treatments on platelet aggregation induced by collagen were examined. Nitrite alone inhibited aggregation, far-red light alone trended to so, while the combination treatment significantly inhibited aggregation compared to control. However, unlike other experiments, the combination treatment was not more effective than nitrite alone.
In our platelet adhesion assay to collagen, far-red light treatment alone trended to decrease adhesion but the result was not significant. Nitrite treatment alone significantly decreased adherence when measured by number of adherent particles, but only trended to do so (not significantly) when using mean fluorescent intensity as a measure. However, the combined treatment consistently decreased platelet adhesion. The fact that far-red light had this effect when combined with nitrite is particularly important given the fact that the blood was continuously flowing past the light in this assay.
Unlike other NO producing agents where RBCs reduce anti-platelet effects, earlier work has shown that the effects of nitrite and light on inhibiting clotting is dependent on the presence of RBCs [14; 18]. We have also shown the additional effects of far-red light require the presence of RBCs in all the clotting assays we measured [24]. The necessity of the RBCs strongly supports the hypothesis that RBCs bioactivate nitrite releasing NO activity. Further support comes from the demonstrations that when an NO scavenger is added, red blood cell-mediated inhibition of platelet activation by nitrite is abrogated [18; 23]. In contrast to these results, one 2016 report purports to show that RBCs do not produce NO bioactivity based on a lack of observed phosphorylated vasodilator-stimulated phosphoprotein (P-VASPSer239), an expected downstream product of NO-mediated production of cGMP in platelets [34]. However, since then, P-VASPSer239 has been detected in the context of red blood cell-mediated inhibition of platelet activation by nitrite [35]. Our results in the current paper further supports the ability of RBCs to bioactivate nitrite.
We have also shown that RBC surface thiols are involved in nitrite bioactivation by RBCs [23] and in the effects of far-red light [24]. Thus, we hypothesized that nitrite bioactivation involves formation of a surface thiol bound NO congener and that far-red light acts by photolyzing this congener (Figure 1). Overall, this hypothesis is partially, but not fully supported by the current work. In all cases nitrite treatment alone decreased platelet activity and other clotting measures, at least as a trend and in most cases significantly; consistent with RBC-mediated bioactivation of nitrite and known effects of NO on clotting. In most, but not all, assays addition of far-red light treatment enhanced the effects of nitrite. The effects of far-red light treatment alone, previously suggested to be due to photolysis of pre-existing thiol bound NO congeners, had weak if any effects in the studies reported here. The lack of individual effects of far-red light monotherapy may be due to low amounts of NO released, complex nature of NO effects on platelets, and differential effects of NO on clotting for different agonists employed as illustrated in Figure 8. On the other hand, it is possible that far-red light acts through an additional or alternative mechanism that also has differential efficacy dependent on agonist used; perhaps involving redox state of mitochondria or thiol status.
One important result from these studies is that the combination therapy of nitrite and far-red light showed universal efficacy even in cases when nitrite alone did not. NO-based therapies for thrombosis have previously been explored [4; 7; 36–41]. We have shown that nitrite is a unique nitric oxide (NO) donating anti-thrombotic agent as its activity is enhanced by red blood cells whereas other NO donors’ activity is blunted by red blood cells [14]. Nitrite is thus potentially the best anti-thrombotic NO donating agent (as blood naturally has red blood cells present). Use of far-red light to enhance the effects of nitrite adds the possibility of localizing the antithrombotic effects when nitrite is administered systemically. The far-red light can penetrate about 0.5 cm of tissue but can also illuminate within the body using an implantable light emitting diode. Another application for the combined light/nitrite treatment is device thrombosis. Device thrombosis is a significant complication in otherwise life-saving interventions including hemodialysis catheters, stents, extracorporeal devices (including those used in cardiopulmonary bypass surgery and extracorporeal membrane oxygenation (ECMO), and hemodialysis), and in left and right ventricular assist devices [1]. Clotting is a major daily problem in continuous renal replacement therapy (CRRT) [42]. Standard care often involves use of systemic anti-coagulant but this can lead to bleeding and, in some cases, heparin-induced thrombocytopenia [2; 3]. Our data, especially that on platelet adhesion in flowing blood, suggests the possibility of systemic nitrite administration and local far-red light illumination as a treatment for device thrombosis. Further work is required to examine this possibility as well as other applications in thrombosis.
These other applications could include COVID-19 where platelet activation and thrombosis contributes to pathology [43; 44].
5. Conclusions
While both nitrite and far-red light illumination have inhibitory effects on platelet activity and clotting, the combination of the two has the most consistent and strongest effects. Mixed results using different agonists and measures of platelet activity may be attributable to the complex, dual role of NO on platelet activation. Further work is warranted to explore the combined nitrite/far-red light treatment of thrombosis.
Highlights.
Nitrite and far-red light effects on clotting and platelet activity were elucidated
Combination treatment was efficacious across all measures
Single treatments showed some variability for different agonists and assays.
Efficacy of combined treatment in treating thrombosis warrants further work.
Funding
This work was supported by the National Institutes of Health [grant number HL098032] and funding from the Translational Science Center, Center for Molecular Signaling, and Center for Redox Biology Medicine at Wake Forest University. We acknowledge services provided by the Flow Cytometry Core Laboratory of the Comprehensive Cancer Center, supported in part by the National Institutes of Health [Grant number P30 CA121291].
Abbreviations:
- ADP
adenosine diphosophate
- RBC
red blood cell
- Hb
hemoglobin
- PRP
platelet rich plasma
- PPP
platelet poor plasma
- PBS
phosphate buffered saline
- LED
light emitting diode
- TRAP
thrombin receptor agonist peptide-6
- MFI
mean fluorescent intensity
- cGMP
cyclic guanosine monophosphate
- P-VASPSer239
phosphorylated vasodilator-stimulated phosphoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sukavaneshvar S, Device thrombosis and pre-clinical blood flow models for assessing antithrombogenic efficacy of drug-device combinations, Advanced Drug Delivery Reviews 112 (2017) 24–34. [DOI] [PubMed] [Google Scholar]
- [2].Selleng S, Selleng K, Heparin-induced thrombocytopenia in cardiac surgery and critically ill patients, Thromb. Haemost 116 (2016) 843–851. [DOI] [PubMed] [Google Scholar]
- [3].Shore-Lesserson L, Baker RA, Ferraris VA, Greilich PE, Fitzgerald D, Roman P, Hammon JW, The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical Practice Guidelines*-Anticoagulation During Cardiopulmonary Bypass, Annals of Thoracic Surgery 105 (2018) 650–662. [DOI] [PubMed] [Google Scholar]
- [4].Radomski MW, Palmer RMJ, Moncada S, THE ROLE OF NITRIC-OXIDE AND CGMP IN PLATELET-ADHESION TO VASCULAR ENDOTHELIUM, Biochem. Biophys. Res. Commun 148 (1987) 1482–1489. [DOI] [PubMed] [Google Scholar]
- [5].Langford EJ, Brown AS, Wainwright RJ, Debelder AJ, Thomas MR, Smith REA, Radomski MW, Martin JF, Moncada S, INHIBITION OF PLATELET ACTIVITY BY S-NITROSOGLUTATHIONE DURING CORONARY ANGIOPLASTY, Lancet 344 (1994) 1458–1460. [DOI] [PubMed] [Google Scholar]
- [6].Moro MA, Russell RJ, Cellek S, Lizasoain I, Su YC, DarleyUsmar VM, Radomski MW, Moncada S, cGMP mediates the vascular and platelet actions of nitric oxide: Confirmation using an inhibitor of the soluble guanylyl cyclase, Proc. Natl. Acad. Sci. USA 93 (1996) 1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stamler JS, Loscalzo J, The Antiplatelet Effects of Organic Nitrates and Related Nitroso-Compounds Invitro and Invivo and Their Relevance to Cardiovascular Disorders, J Am Coll Cardiol 18 (1991) 1529–1536. [DOI] [PubMed] [Google Scholar]
- [8].Keaney JF, Loscalzo J, Diabetes, oxidative stress, and platelet activation, Circulation 99 (1999) 189–191. [DOI] [PubMed] [Google Scholar]
- [9].Loscalzo J, Nitric oxide insufficiency, platelet activation, and arterial thrombosis, Circ. Res 88 (2001) 756–62. [DOI] [PubMed] [Google Scholar]
- [10].Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, Qin X, The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model, Blood 116 (2010) 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schafer A, Wiesmann F, Neubauer S, Eigenthaler M, Bauersachs J, Channon K, Rapid Regulation of Platelet Activation In Vivo by Nitric Oxide, Circulation 109 (2004) 1819–1822. [DOI] [PubMed] [Google Scholar]
- [12].Badimon L, Vilahur G, Nitric Oxide Donors as Platelet Inhibitors in: Freedman J, Loscalzo J, (Eds.), New Therapeutic Agents in Thrombosis and Thrombolysis, CRC Press, Boca Raton, Fl, 2009, pp. 499–516. [Google Scholar]
- [13].Goudie MJ, Pant J, Handa H, Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices, Scientific Reports 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wajih N, Basu S, Jailwala A, Kim HW, Ostrowski D, Perlegas A, Bolden CA, Buechler NL, Gladwin MT, Caudell DL, Rahbar E, Alexander-Miller MA, Vachharajani V, Kim-Shapiro DB, Potential therapeutic action of nitrite in sickle cell disease, Redox Biol 12 (2017) 1026–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Doyle MP, Hoekstra JW, Oxidation of Nitrogen-Oxides by Bound Dioxygen in Hemoproteins, J Inorg Biochem 14 (1981) 351–358. [DOI] [PubMed] [Google Scholar]
- [16].Eich RF, Li TS, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS, Mechanism of NO-induced oxidation of myoglobin and hemoglobin, Biochemistry-US 35 (1996) 6976–6983. [DOI] [PubMed] [Google Scholar]
- [17].Herold S, Exner M, Nauser T, Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin, Biochemistry-US 40 (2001) 3385–3395. [DOI] [PubMed] [Google Scholar]
- [18].Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N, Platelet Inhibition by Nitrite Is Dependent on Erythrocytes and Deoxygenation, PLOS One 7 (2012) e30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hall CN, Garthwaite J, What is the real physiological NO concentration in vivo?, Nitric Oxide-Biol Ch 21 (2009) 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Giustarini D, Rossi R, Plasma S-nitrosothiols and chronic renal failure, American Journal of Physiology-Renal Physiology 287 (2004) F1294–F1294. [DOI] [PubMed] [Google Scholar]
- [21].Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD, Acute effect of a high nitrate diet on brain perfusion in older adults, Nitric Oxide 24 (2011) 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brooks J, The action of nitrite on Haemoglobin in the absence of oxygen, Proc. Royal Soc. London - Series B, Biol. Sci 123 (1937) 368–382. [Google Scholar]
- [23].Wajih N, Liu X, Shetty P, Basu S, Wu H, Hogg N, Patel RP, Furdui CM, Kim-Shapiro DB, The role of red blood cell S-nitrosation in nitrite bioactivation and its modulation by leucine and glucose, Redox Biol 8 (2016) 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wajih N, Basu S, Ucer KB, Rigal F, Shakya A, Rahbar E, Vachharajani V, Guthold M, Gladwin MT, Smith LM, Kim-Shapiro DB, Erythrocytic bioactivation of nitrite and its potentiation by far-red light, Redox Biology 20 (2019) 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pieters M, Philippou H, Undas A, de Lange Z, Rijken DC, Mutch NJ, An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH, Journal of Thrombosis and Haemostasis 16 (2018) 1007–1012. [DOI] [PubMed] [Google Scholar]
- [26].Whyte CS, Mitchell JL, Mutch NJ, Platelet-Mediated Modulation of Fibrinolysis, Seminars in Thrombosis and Hemostasis 43 (2017) 115–128. [DOI] [PubMed] [Google Scholar]
- [27].Smolenski A, Novel roles of cAMP/cGMP-dependent signaling in platelets, Journal of Thrombosis and Haemostasis 10 (2012) 167–176. [DOI] [PubMed] [Google Scholar]
- [28].Estevez B, Du XP, New Concepts and Mechanisms of Platelet Activation Signaling, Physiology 32 (2017) 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lindkvist M, Fernberg U, Ljungberg LU, Falker K, Fernstrom M, Hurtig-Wennlof A, Grenegard M, Individual variations in platelet reactivity towards ADP, epinephrine, collagen and nitric oxide, and the association to arterial function in young, healthy adults, Thromb. Res 174 (2019) 5–12. [DOI] [PubMed] [Google Scholar]
- [30].Irwin C, Roberts W, Naseem KM, Nitric oxide inhibits platelet adhesion to collagen through cGMP-dependent and independent mechanisms: The potential role for S-nitrosylation, Platelets 20 (2009) 478–486. [DOI] [PubMed] [Google Scholar]
- [31].Riba R, Sharifi M, Farndale R, Naseem KM, Regulation of platelet guanylyl cyclase by collagen: evidence that Glycoprotein VI mediates platelet nitric oxide synthesis in response to collagen, Thromb. Haemost 94 (2005) 395–403. [DOI] [PubMed] [Google Scholar]
- [32].Wang GR, Zhu Y, Halushka PV, Lincoln TM, Mendelsohn ME, Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase, Proc. Natl. Acad. Sci. USA 95 (1998) 4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gambaryan S, Friebe A, Walter U, Does the NO/sGC/cGMP/PKG pathway play a stimulatory role in platelets?, Blood 119 (2012) 5335–5336. [DOI] [PubMed] [Google Scholar]
- [34].Gambaryan S, Subramanian H, Kehrer L, Mindukshev I, Sudnitsyna J, Reiss C, Rukoyatkina N, Friebe A, Sharina I, Martin E, Walter U, Erythrocytes do not activate purified and platelet soluble guanylate cyclases even in conditions favourable for NO synthesis, Cell Communication and Signaling 14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Parakaw Tipparat, Suknunth Kran, Vivithanaporn Pornpun, Schlagenhauf Axel, Topanurak Supachai, Fucharoen Suthat, Pattanapanyasat Kovit, Schechter Alan, Sibmooh Nathawut, S. Srihirun, Platelet inhibition and increased phosphorylated vasodilator-stimulated phosphoprotein following sodium nitrite inhalation, Nitric Oxide 66 (2017) 10–16. [DOI] [PubMed] [Google Scholar]
- [36].Loscalzo J, Nitric oxide insufficiency, platelet activation, and arterial thrombosis, Circ. Res 88 (2001) 756–762. [DOI] [PubMed] [Google Scholar]
- [37].Voetsch B, Jin RC, Loscalzo J, Nitric oxide insufficiency and atherothrombosis, Histochem. Cell Biol 122 (2004) 353–367. [DOI] [PubMed] [Google Scholar]
- [38].Vilahur G, Baldellou MI, Segales E, Salas E, Badimon L, Inhibition of thrombosis by a novel platelet selective S-nitrosothiol compound without hemodynamic side effects, Cardiovasc. Res 61 (2004) 806–816. [DOI] [PubMed] [Google Scholar]
- [39].Vilahur G, Badimon L, Nitric Oxide Donors as Platelet Inhibitors in: Freedman JE, Loscalzo J, (Eds.), New Therapeutic Agents in Thrombosis and Thrombolysis, Informa Healthcare, New York, 2009, pp. 499–516. [Google Scholar]
- [40].Karmohapatra SK, Chakraborty K, Kahn NN, Sinha AK, The role of nitric oxide in aspirin induced thrombolysis in vitro and the purification of aspirin activated nitric oxide synthase from human blood platelets, Am J Hematol 82 (2007) 986–995. [DOI] [PubMed] [Google Scholar]
- [41].Jana P, Maiti S, Kahn NN, Sinha AK, Estriol-induced fibrinolysis due to the activation of plasminogen to plasmin by nitric oxide synthesis in platelets, Blood Coagul. Fibrinolysis 26 (2015) 316–323. [DOI] [PubMed] [Google Scholar]
- [42].Joannidis M, Straaten H, Clinical review: Patency of the circuit in continuous renal replacement therapy, Crit Care 11 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Connors J, Levy J, COVID-19 and its implications for thrombosis and anticoagulation, Blood 135 (2020) 2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben CJ, Petrey AC, Tolley ND, Guo L, Cody MJ, Weyrich AS, Yost CC, Rondina MT, Campbell RA, Platelet Gene Expression and Function in COVID-19 Patients, Blood (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


