Abstract
Cognitive-behavioral therapy (CBT), a first-line treatment for pediatric anxiety disorders, is based on principles of threat learning and extinction. However, CBT does not work sufficiently for up to 40% of clinically anxious youth. The neural and behavioral correlates of conditioned inhibition might provide promising targets for attempts to improve CBT response. During conditioned inhibition, threat and safety cues appear together, forming a safety compound. Here, we test whether this safety compound elicits a reduced fear response compared to pairing the threat cue with a novel cue (novel compound). The current pilot study compares behavioral, physiological, and neural correlates of conditioned inhibition between children with (n=17, Mage=13.09, SD=3.05) and without (n=18, Mage=14.49, SD=2.38) anxiety disorders. Behavioral and physiological measures did not differ between children with and without anxiety disorders during fear acquisition. During testing, children with anxiety disorders showed overall higher skin conductance response and expected to hear the aversive sound following the novel compound more often than children without anxiety disorders. Children with anxiety disorders showed more activity in the right ventromedial prefrontal cortex (vmPFC) to the safety versus novel compound. Children without anxiety disorders showed the opposite pattern - more right vmPFC activity to the novel versus safety compound (F(1,31)=5.40, p=0.03). No group differences manifested within the amygdala, dorsal anterior cingulate cortex, or hippocampus. These pilot findings suggest a feasible approach for examining conditioned inhibition in pediatric anxiety disorders. If replicated in larger samples, findings may implicate perturbed conditioned inhibition in pediatric anxiety disorders and provide targets for CBT.
Keywords: anxiety, conditioned inhibition, exposure, fear acquisition, development
1. Introduction
CBT1, a first-line treatment, fails to produce remission in at least 40% of anxious children [1, 2]. Since CBT applies threat-learning principles, translational neuroscience research on threat learning and extinction informs attempts to target CBT-resistant anxiety [3, 4]. Findings from animal studies suggest that CBT might be improved by extending research on conditioned inhibition, which occurs when threat and safety cues are processed simultaneously [5, 6]. However, minimal research examines conditioned inhibition in pediatric anxiety disorders. To launch this line of research, we compare behavioral, physiological, and neural correlates of conditioned inhibition in children with and without anxiety disorders to gain more insight into conditioned inhibition as a potential mechanism for improving CBT.
Pediatric anxiety disorders are common and impactful [7–11], and often emerge during late childhood or early adolescence [12]. It is important to study mechanisms underlying anxiety, such as threat learning, in youth in an attempt to interrupt cycles leading to chronic anxiety. In threat learning paradigms, a previously-neutral threat cue (conditioned stimulus, CS+) is repeatedly paired with an aversive stimulus (typically electric shock or loud noise, the UCS), whereas a safety cue (CS−) is never paired with the aversive stimulus [13]. During fear acquisition, differentiating between threat and safety cues engages the amygdala, dACC, hippocampus, and vmPFC [14, 15]. During such paradigms, adults with anxiety disorders show an increased fear response (measured via subjective ratings of anxiety, SCR, or startle response) to safety cues [16], and children with anxiety disorders show an increased fear response (i.e., subjective anxiety, SCR, or startle response) to both threat and safety cues [17] during fear acquisition relative to age-matched participants without anxiety disorders.
Another important process in threat learning is extinction. During extinction, the threat cue is no longer paired with the aversive stimulus, thereby forming a new association between the threat cue and the absence of the aversive stimulus. As a result, the subsequent fear response to the threat cue is diminished. Extinction is related to activity in brain regions such as the amygdala, dACC, and vmPFC [18, 19]. Adults with anxiety disorders display increased fear responses to the threat cue [16], and children with anxiety disorders show increased fear responses to both threat and safety cues [17] compared to age-matched participants without anxiety disorders. CBT-based exposure is based on these processes of threat learning and extinction [20]. Therefore, research on such processes informs attempts to treat CBT-resistant anxiety in children [1, 2].
Conditioned inhibition supports fear reduction in ways that differ from extinction. During conditioned inhibition, threat and safety cues are presented simultaneously as a safety compound to test whether the safety cue reduces the fear response to the threat cue. Unlike extinction memories, which compete with an earlier threat memory evoked by a single cue, safety cues in conditioned inhibition are never paired with the aversive stimulus [5, 21]. Animal studies have shown a reduction in the fear response to a safety compound relative to a threat cue presented by itself [5, 6]. Behavioral evidence in rodents further suggests that fear reduction via conditioned inhibition may be less susceptible to the effects of prior stress than extinction [22], particularly during adolescence [23]. In studies with humans, a novel compound (i.e., threat cue paired with a novel cue) is often included as a control condition to test whether the safety stimulus (presented during the acquisition phase) reduces the fear response over and above a novel stimulus (not yet presented during the task). Indeed, healthy adults show reduced fear responses to a safety compound compared to a novel compound [24]. In contrast, adults with PTSD show less fear reduction in response to the safety compound, suggesting deficient conditioned inhibition [25]. To date, only one study has investigated the neural correlates of conditioned inhibition in humans. Specifically, findings demonstrated that the ventral hippocampus interacts with the dACC in both mice and healthy adults during conditioned inhibition to support fear reduction [26].
Conditioned inhibition is particularly relevant for pediatric anxiety disorders. Threat and safety learning undergo marked changes during development [27–29], as does the neural circuitry that supports these processes [30, 31]. Cross-species research finds reduced fear extinction in adolescents compared to juveniles and adults, and this effect is related to reduced vmPFC synaptic plasticity in mice [32]. Moreover, as compared to adults, adolescents also show higher amygdala reactivity and delayed vmPFC engagement during extinction learning [33] and altered vmPFC activation and amygdala-vmPFC connectivity during extinction recall [34, 35]. Conditioned inhibition may be more dependent on a broader network of regions [21], including the hippocampus and dACC [26], that develop later in life [36]. Delineating the neural mechanisms supporting conditioned inhibition during development and examining how they may differ in youth with anxiety disorders is a critical next step in translating research on conditioned inhibition.
The current pilot study compares behavioral, physiological, and neural correlates of conditioned inhibition between youth with and without anxiety disorders. Participants performed a conditioned inhibition task [26] in the MRI scanner while we continuously measured SCR. We hypothesized that children with anxiety disorders would show differential behavioral, physiological, and neural responses during fear acquisition and conditioned inhibition compared to children without anxiety disorders. Given the preliminary nature of the current study, we did not specify a direction for the hypothesized group differences. We focused on the threat versus safety cue contrast for fear acquisition and on the safety compound versus novel compound contrast for conditioned inhibition. In light of prior research on aberrant threat and safety learning in anxiety disorders, our analyses focused on group differences in neural response in the amygdala, dACC, hippocampus, and vmPFC [14, 18, 26, 34, 35, 37–42]. As this was the first study of conditioned inhibition in youth, we also performed a whole-brain analysis to characterize broader differences between children with and without anxiety disorders in brain activity during fear acquisition and conditioned inhibition.
2. Methods
2.1. Participants
Participants were 35 children between 8 and 18 years of age with anxiety disorders (n = 17; Mage = 13.09 years, SD = 3.05) and without anxiety disorders (n = 18; Mage = 14.49 years, SD = 2.38; see Table 1 for descriptive statistics). Anxiety disorders were diagnosed by a licensed psychiatric nurse or psychologist and confirmed by a psychiatrist using the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version [43]. Fourteen children had a diagnosis of generalized anxiety disorder, 10 of social anxiety disorder, 8 of specific phobia, and 7 of separation anxiety disorder (most children had multiple diagnoses). All children with anxiety disorders were treatment-seeking, had a CGI severity score [44] of 3 or higher (“mildly ill” or more), and were medication-free. The children without anxiety disorders were free of any current psychiatric diagnosis and had a CGI severity score of 1 (“normal, not at all ill”). Across groups, exclusion criteria were a history of significant medical or neurological disorder, current suicidal ideation, diagnosis of attention-deficit/hyperactivity disorder of sufficient severity to require pharmacotherapy, Tourette’s Disorder, obsessive-compulsive disorder, PTSD, conduct disorder, major depressive disorder, past or current history of mania, psychosis, severe pervasive developmental disorder, IQ below 70, colorblindness, or any MRI contraindications (such as braces, metal implants, claustrophobia, etc.). An additional 11 participants (6 children with anxiety disorders, 5 children without anxiety disorders) were excluded from analyses. Of those, 10 children were excluded because they discontinued the task (2 due to technical problems, 5 complained about the aversive sound, 2 fell asleep, 1 reported discomfort); these children had to be excluded because they did not have distortion correction scans, which were acquired later in the scanning protocol2. One child was excluded due to excessive motion in fMRI data (see below for criteria). All procedures were approved by the Institutional Review Board of the National Institute of Mental Health. Legal guardians of participants provided written informed consent, and children provided written assent to participate.
Table 1.
Descriptive statistics of children with and without anxiety disorders.
| Children with AD | Children without AD | Test statistic | p | |
|---|---|---|---|---|
| Girls/boys | 11/6 | 14/4 | X2(1)=0.73 | 0.39 |
| Age (mean, SD) | 13.09 (3.05) | 14.49 (2.38) | F(1,33)=2.32 | 0.14 |
| IQ (mean, SD) | 115.06 (11.83) | 104.71 (11.74) | F(1,31)=6.36 | 0.02 |
| SCARED-avg total (mean, SD) | 27.16 (10.96) | 4.56 (2.83) | F(1,33)=71.61 | <0.001 |
| STAI trait (mean, SD) | 36.24 (7.03) | 25.11 (4.66) | F(1,33)=30.76 | <0.001 |
| Race | ||||
| American Indian or Alaskan | ||||
| Native | 1 | 0 | ||
| Black or African American | 0 | 8 | ||
| Multiple races | 1 | 4 | ||
| Unknown | 2 | 1 | ||
| White | 13 | 5 | ||
| Highest parental education | ||||
| Graduate professional degree (masters or above) | 13 | 6 | ||
| Standard college graduation | 2 | 5 | ||
| Partial college (1 year or more) | 0 | 3 | ||
| Household gross income | ||||
| $15,000 – $24,999 | 0 | 3 | ||
| $25,000 – $39,999 | 0 | 0 | ||
| $40,000 – $59,999 | 0 | 1 | ||
| $60,000 – $89,999 | 4 | 2 | ||
| $90,000 – $179,999 | 3 | 6 | ||
| over $180,000 | 6 | 2 |
Note: Not enough data were available to test differences between the groups in the distribution of race, highest parental education, and household gross income. IQ was missing for 2 participants; highest parental education was missing for 6 participants; household gross income was missing for 8 participants.
AD = anxiety disorders; SCARED = Screen for Child Anxiety Related Disorders; STAI = State-Trait Anxiety Inventory
Table 1 shows the descriptive statistics of the two groups. Age and the distribution of sex assigned at birth were similar across the groups (age: F(1, 33) = 2.32, p = 0.14; sex: X2(1) = 0.73, p = 0.39). IQ (as measured by the Wechsler Abbreviated Scale of Intelligence; [45]) differed between diagnostic groups (F(1, 31) = 6.36, p = 0.02). Compared to children without anxiety disorders, children with anxiety disorders had higher current anxiety symptoms (F(1, 33) = 71.61, p < 0.001), as measured by the SCARED [46], and higher trait anxiety (F(1, 33) = 30.76, p < 0.001), as measured by the STAI [47].
2.2. Current anxiety symptom severity
In addition to categorical diagnosis of an anxiety disorder, current severity of anxiety symptoms was assessed continuously for all groups, regardless of diagnosis, with the 41-item child- and parent-report versions of the SCARED [46]. Both the self-reported SCARED (M = 9.94 days, SD = 15.05) and the parent-reported SCARED (M =15.06 days, SD = 23.82) were administered within three months of the scan. In cases of missing items, these were replaced with the participant’s average value for the other items for up to two missing items. Total scores from the child- and parent-report versions of the SCARED were averaged for each participant to mitigate reporter discrepancy [48, 49].
2.3. Conditioned Inhibition Task
2.3.1. Task design.
Participants completed a conditioned inhibition task in the MRI scanner [26] which comprised four phases: acquisition, testing, extinction, and reversal (Figure 1a). Throughout the task, participants were presented with several basic, colored geometric shapes. Participants were informed that they would periodically hear an aversive sound and that they might be able to determine when they would hear the sound. The aversive sound was a loud white noise (~100 dB) presented via headphones (mkII+ MR Confon, Cambridge Research Systems, Kent, UK). Children wore earplugs to reduce the noise from the scanner. Before the task started, children listened to a different audio file (a voice saying, “Do you think you will hear the sound?”) to make sure the volume was tolerable. If children indicated that the volume was tolerable, the volume was increased by a standard unit up to three times.
Figure 1.
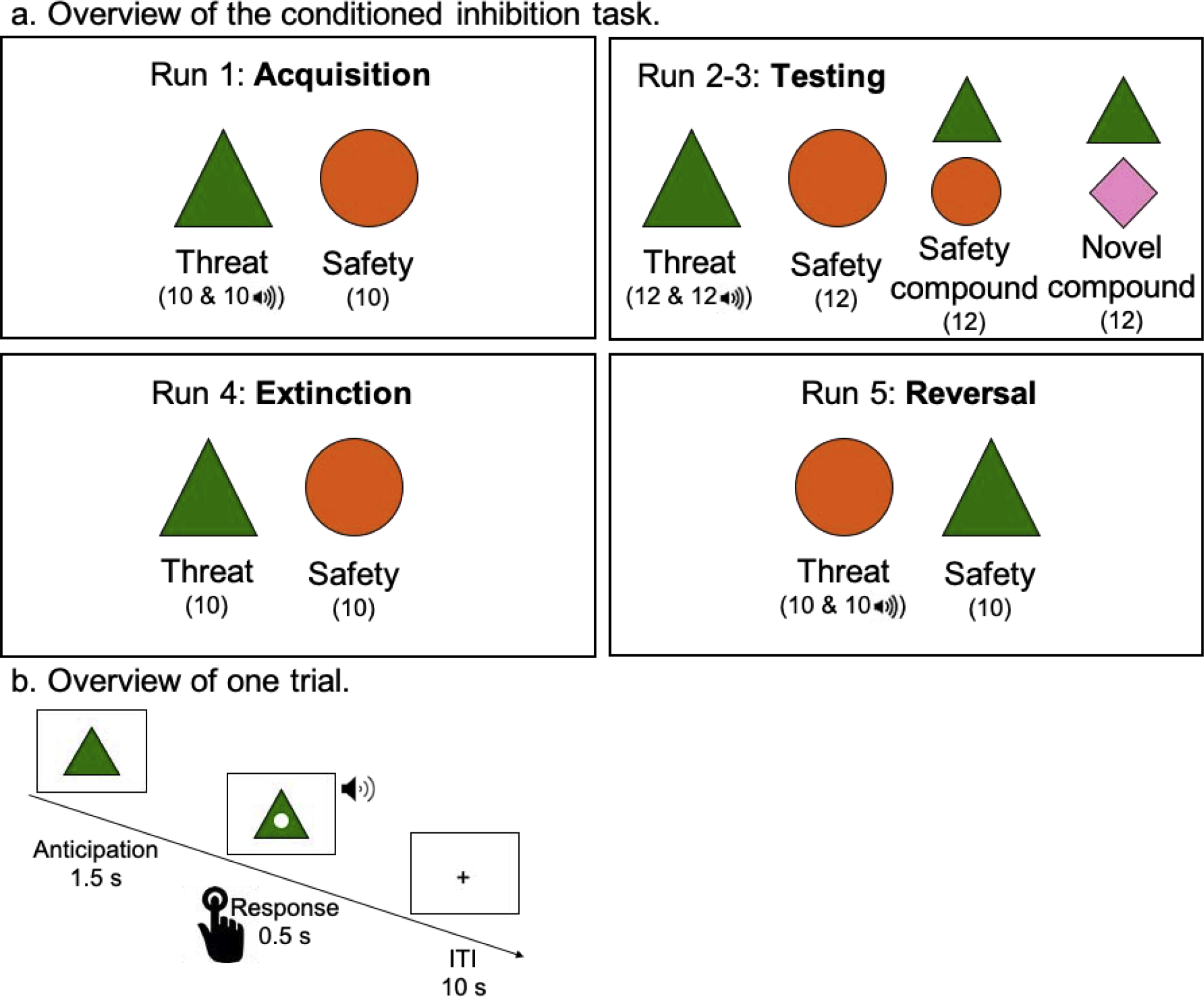
Overview of the Conditioned Inhibition Task.
Note: The number of trials is displayed in parentheses; ITI = inter-trial interval.
In the acquisition phase, participants were presented with a threat cue that was paired with the aversive sound 50% of the time (in randomized order) and a safety cue that was never paired with the aversive sound. Stimuli were presented in a blocked design during this acquisition phase, and the order of threat and safety blocks was randomized. In the testing phase, two stimulus compounds were added to the stimuli presented: the threat cue paired with the safety cue (safety compound) and the threat cue paired with a novel cue (novel compound). The novel compound served as a control condition, to test whether the safety compound reduced the fear response over and above a novel stimulus and to control for the possibility of external inhibition. Neither compound stimulus was ever presented with the aversive sound. During the testing phase (split into two fMRI runs due to time constraints) participants viewed the threat cue with the aversive sound, threat cue without aversive sound, safety cue, safety compound, and novel compound each six times per run in randomized order. During the extinction phase, participants were presented with the threat and safety cues in a random order; neither cue was paired with the aversive sound. During the reversal phase, cue contingencies were reversed such that the previous safety cue was paired with the aversive sound 50% of the time (in randomized order) and the previous threat cue was never paired with the aversive sound. Like the acquisition phase, the stimuli were presented in a blocked design, and the order of threat and safety blocks was randomized.
Each trial (Figure 1b) started with the shape in the center of the screen. After 1.5 seconds, a white dot appeared in the center of the shape for 0.5 seconds. In order to ensure that participants were paying attention, they were instructed to press a button when this dot appeared. In trials where the threat cue was paired with the aversive sound, the sound was presented for 0.5 seconds at the same time as the white dot. Participants viewed a fixation cross for 10 seconds between trials. The task was programmed and administered using E-Prime software (Psychology Software Tools, Pittsburgh, PA).
2.3.2. Behavioral measures.
Participants were asked to indicate if they expected to hear the aversive sound (yes/no) for each type of stimulus after every run, and two additional times during each testing run. After the MRI scan, participants were again asked if they expected to hear the aversive sound (yes/no) for each stimulus. In addition, participants were asked to press a button when a dot appeared in the stimulus. This was primarily to ensure that participants were paying attention, but we also explored whether reaction time differed between stimuli and groups.
2.4. Skin conductance response data acquisition and preprocessing
SC data were continuously collected during the fMRI scan with two electrodes on either two fingers or the foot using a Biopac MRI-compatible system (Biopac Systems Inc, Goleta, CA) with a sampling rate of 1000 Hz and analyzed with AcqKnowledge 4 software (Biopac Systems Inc, Goleta, CA). SC data were filtered with a 3 Hz low-pass filter. For each stimulus separately, the minimum SC value during baseline (defined as the 1-second window before stimulus presentation [i.e., the 1 second prior to the onset of the anticipation period]) and the maximum SC value during a 7-second window starting with stimulus presentation were calculated [13]. Outliers in SC values (more than three standard deviations from the mean) were replaced with the mean SC across all stimuli in the task within each participant. To calculate SCR per trial, the baseline SC value was subtracted from the maximum SC value per trial; negative values were replaced with a value of zero (the mean number of zero values per condition per phase of the task for children with and without anxiety disorders are shown in Supplementary Table A2). Then, SCR values were averaged for each stimulus (for threat cues, only non-reinforced trials were included to avoid contamination by the US) per phase of the task [50]; no transformation was applied. SCR values per trial during the acquisition phase are shown in Supplementary Figure A1.
2.5. Statistical analyses
Our goal was to compare expectancies, reaction time, SCR, and neural correlates of conditioned inhibition between children with and without anxiety disorders. Effects of age and age-squared are reported in supplementary material B. Therefore, we focused on the findings of the acquisition and testing phases, and the findings of the extinction and reversal phases are reported in supplementary material C. Consistent with prior work [13], only non-reinforced trials were included in the analysis of threat cues.
Chi-square analyses were conducted to compare children with and without anxiety disorders on their expectations about hearing the aversive sound. These chi-square analyses were conducted per stimulus (threat, safety, safety compound, and novel compound), testing the group (with or without anxiety disorders) by expectancy (yes or no) interaction. For the acquisition phase, two repeated-measures ANOVAs were conducted with stimulus (threat, safety) as a within-subject factor, age and age-squared as covariates, group (with or without anxiety disorders) as a between-subjects factor, and either reaction time or SCR as the dependent variable. For the testing phase, two repeated-measures ANOVAs were conducted with stimulus (threat, safety, safety compound, novel compound) and timing (first versus second testing run) as within-subject factors, age and age-squared as covariates, group (with or without anxiety disorders) as a between-subjects factor, and either reaction time or SCR as the dependent variable. We used IBM SPSS Statistics Subscription Version 26.0 (IBM Corp., Armonk, NY) for these analyses, and alpha was set at 0.05. Supplementary Table A1 shows the number of missing values for behavioral and physiological variables for children with and without anxiety disorders.
2.6. Neuroimaging Data Acquisition and Analyses
2.6.1. Data acquisition.
Neuroimaging data were acquired on a 3T MR750 General Electric scanner (Waukesha, Wisconsin, USA) with a 32-channel head coil. The conditioned inhibition task was completed across 5 functional runs of varying lengths (196, 214, 214, 136, and 196 volumes, respectively). T2*-weighted echo-planar images with 47 contiguous interleaved axial slices were collected bottom-up to measure BOLD signal (TR = 2000 ms; TE = 30 ms; flip angle = 70; FOV = 240 mm; matrix = 96×96; in-plane resolution = 2.5×2.5×3 mm). We acquired two additional scans with the same parameters as the functional images for distortion correction: one with 10 volumes acquired in the same direction (bottom-up) and one with 10 volumes acquired in the opposite direction (top-down) as the functional images. We also collected a whole-brain, high-resolution, T1-weighted anatomical scan on the same day (MPRAGE; TE = min full; TI = 900 ms; flip angle = 7; FOV = 256 mm; matrix = 256 × 256; in plane resolution = 1×1×1 mm).
2.6.2. Individual level analysis.
Data were processed and analyzed using FSL version 5.0.11 [51]. Preprocessing of each individual’s data included skull stripping [52], removing the first four volumes to correct for steady-state effects, motion correction using MCFLIRT [53], and distortion correction using topup [51, 54]. We used FSL FEAT [55] with MCFLIRT motion correction, slice timing correction, spatial smoothing with a 6 mm Gaussian kernel (FWHM), registration from functional scans to structural scan, and nonlinear transformation into standard MNI space. Then we employed ICA-AROMA [56] for motion correction. Finally, another FSL FEAT was conducted with the non-aggressive denoised output from ICA-AROMA with high-pass temporal filtering, registration from functional scans to structural scan, nonlinear transformation into standard MNI space, and FILM prewhitening.
An individual-level general linear model was conducted including the following regressors: each of the stimuli with a temporal derivative and convolved with the double-gamma hemodynamic response function (for testing phase: threat cue with aversive sound, threat cue without aversive sound, safety cue, safety compound, novel compound, expectancy questions at end of the run), nuisance regressors (i.e., signal from brain stem, corpus callosum, cerebral spinal fluid, white matter, and cerebellum), and time points to be removed because of motion (see below). Consistent with prior work, temporal derivatives and nuisance regressors were added in FSL FEAT [55] to improve model fit and to reduce unexplained noise3.
In order to minimize the potential effects of motion on task-related results, strict motion correction was implemented. Participants with a mean FD [57] of 0.5 mm or higher were excluded (n = 1). Furthermore, TR pairs with particularly large motion were identified by FSL’s fsl_motion_outliers function, defining an outlier as falling 1.5 times the interquartile range above the upper quartile and with FD as the motion metric. Each outlier TR was included in a confound matrix that was added to the subject’s individual-level design matrix. Thus, the effects of these TRs with large motion were regressed out of the results while maintaining the temporal structure of the timeseries. Within this sample, a mean of 6.52% (SD = 1.83%) and a total range of 0.48 – 14.58% of TRs were regressed out of individual-level analyses. The number of TRs that were regressed out and the mean FD did not differ between children with and without anxiety disorders in any of the runs (Fs < 2.06, ps > 0.16)4.
2.6.3. ROI group analysis.
Based on prior research on threat and safety learning [14, 18, 34, 35, 37, 38] and consistent with research on conditioned inhibition in adults [26], the amygdala, dACC, hippocampus, and vmPFC were isolated as ROIs. The amygdala was defined using the Harvard-Oxford atlas in FSL [58] (left amygdala: 336 voxels; right amygdala: 341 voxels). The dACC was defined based on the bilateral anterior cingulate and paracingulate regions from the Harvard-Oxford atlas in FSL and divided into subregions at the genu of the corpus callosum [26, 59] (4354 voxels). In line with previous work on conditioned inhibition [26, 39–42], we focused on the anterior hippocampus. The anterior hippocampus was defined probabilistically based on an in-house database of manual segmentations from Hindy and Turk-Browne [60] (left hippocampus: 226 voxels; right hippocampus: 258 voxels). The anterior vmPFC was defined based on the Mackey vmPFC atlas [61] (left vmPFC: 831 voxels; right vmPFC: 831 voxels). Mean percent signal change was extracted from each region using FSL’s featquery tool for the threat versus safety contrast in the acquisition phase, and for the safety compound versus novel compound contrast in the testing phase. These percent signal change values were compared between children with and without anxiety disorders with an ANOVA with group (with and without anxiety disorders) as a between-subjects factor and age and age-squared as covariates, separately for the left and right hemispheres.
2.6.4. Whole-brain group analysis.
Given that this is the first study using this paradigm in a developmental sample, we also conducted group-level analyses to test for whole-brain differences between children with and without anxiety disorders. We focused on the threat versus safety contrast in the acquisition phase and on the safety compound versus novel compound contrast in the testing phase. Group (with or without anxiety disorders), age, and age-squared were included as explanatory variables in the model. The contrasts of interest were the effects of group (with>without anxiety disorders, without>with anxiety disorders), age (positive association, negative association), and age-squared (positive association, negative association). The analyses were conducted on the regression coefficients from the individual-level analyses using FMRIB’s Local Analysis of Mixed Effects 1 [62]. Inference used the cluster extent test statistic, with a cluster-forming threshold of Z > 3.1; clusters were considered significant if FWER-corrected p-value ≤ 0.05. Since this is an exploratory study, we also report effects with a cluster-forming threshold of Z > 2.3 and pFWER ≤ 0.05. Results from threat cue versus safety compound contrast are reported in supplementary material D.
3. Results
3.1. Acquisition phase
3.1.1. Expectancies and reaction time.
Most children (64.5%) reported that they expected to hear the aversive sound with the threat cue at the end of the acquisition phase. Most children (86.7%) also expected to hear the aversive sound with the safety cue. However, for unknown reasons, 20 participants did not respond to the expectancy question for the safety cue, so this result is based on only 15 participants. Children with and without anxiety disorders did not differ in how often they expected to hear the aversive sound with the threat cue (X2(1) = 0.61, p = 0.48) or with the safety cue (X2(1) = 0.10, p > 0.99; Figure 2a). After the MRI scan, almost all children reported that they expected to hear the aversive sound with the threat cue (87.1%) and not with the safety cue (87.1%) in the beginning of the task. Children with and without anxiety disorders did not differ in how often they expected to hear the aversive sound with the threat cue (X2(1) = 2.06, p = 0.28) or with the safety cue (X2(1) = 0.12, p > 0.99) when asked after the scan (Figure 2b). Reaction times did not differ between children with and without anxiety disorders (F(1, 29) = 1.51, p = 0.23) or between threat and safety cues (F(1, 29) = 3.16, p = 0.09). There was also no interaction between group and stimulus (F(1, 29) = 0.82, p = 0.37; Figure 2c).
Figure 2.
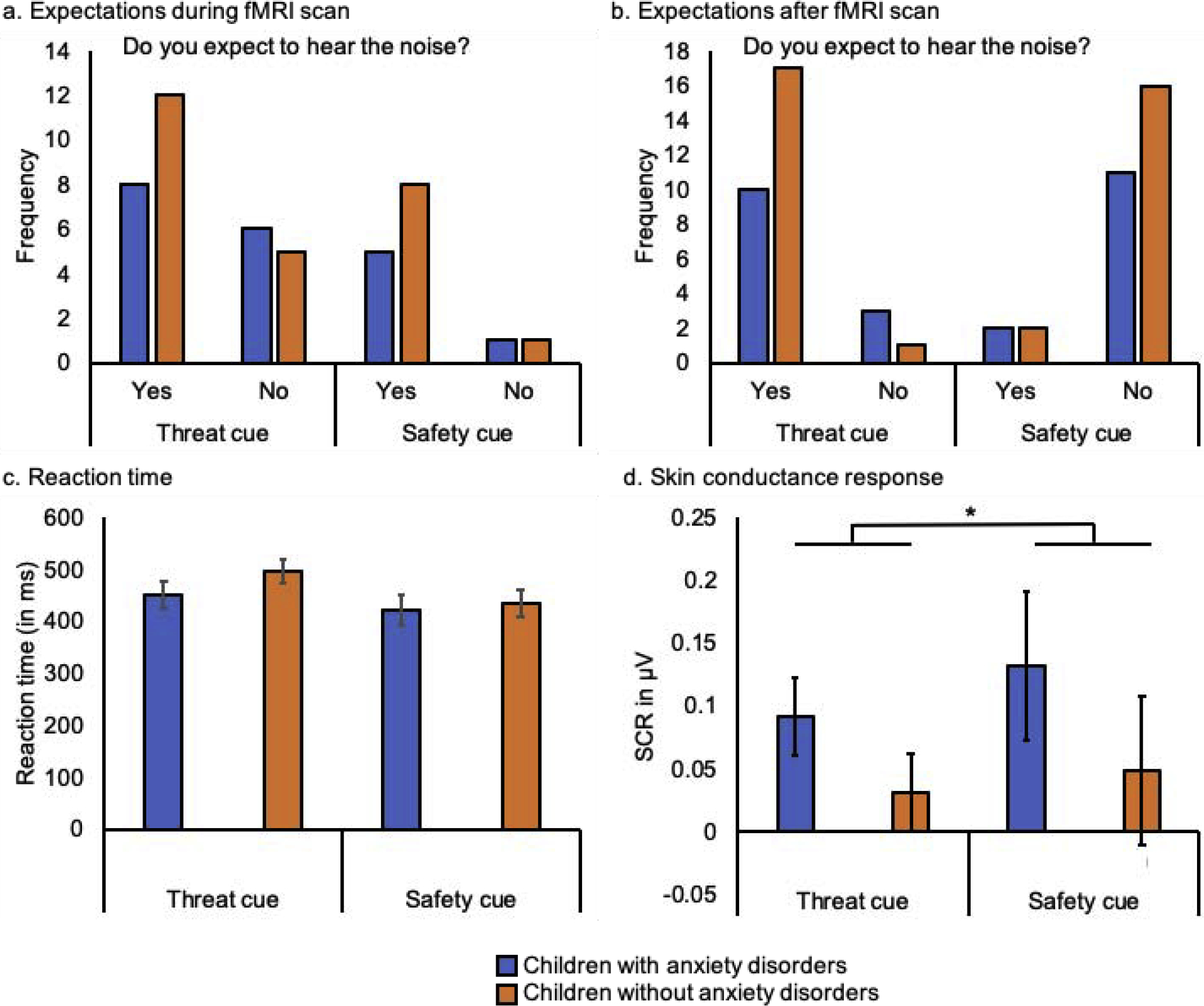
Behavioral and physiological responses to threat and safety cues during the acquisition phase of the conditioned inhibition task in children with and without anxiety disorders: (a) expectancy ratings after the acquisition phase during the fMRI scan, (b) expectancy ratings about the acquisition phase collected after the fMRI scan, (c) reaction time, and (d) skin conductance response (SCR). Error bars display +/− 1 standard error.
3.1.2. Skin conductance response.
Overall, children with and without anxiety disorders showed a higher SCR to the safety cue than to the threat cue during acquisition (F(1, 28) = 6.17, p = 0.02). There was no difference in mean SCR between children with and without anxiety disorders (F(1, 28) = 1.49, p = 0.23), nor an interaction between stimulus and group (F(1, 28) = 0.32, p = 0.58; Figure 2d).
3.1.3. ROI results.
On average, children showed more activity to the safety cue compared to the threat cue in the left hippocampus (t(34) = −2.74, p = 0.01) and the left vmPFC (t(34) = −2.04, p = 0.049). There were no significant differences between children with and without anxiety disorders in activity in the amygdala, dACC, hippocampus, or vmPFC to the threat versus safety cues during the acquisition phase (Fs < 0.71, ps > 0.41).
3.1.4. Whole-brain results.
There were no significant effects at the cluster-forming threshold of Z >3.1. On average, brain activity in response to threat versus safety cues did not differ across all children. There were also no differences between children with and without anxiety disorders.
3.2. Testing phase
3.2.1. Expectancies and reaction time.
100% of the children expected to hear the aversive sound paired with the threat cue after the testing phase. 40% of children expected to hear the aversive sound paired with the safety cue, 43.9% with the safety compound, and 34.3% with the novel compound. Children with and without anxiety disorders did not differ in how often they expected to hear the aversive sound with the safety cue (X2(1) = 2.31, p = 0.18) and with the safety compound (X2(1) = 0.24, p = 0.74). Most children without anxiety disorders did not expect to hear the aversive sound with the novel compound (83.3%), which was not the case for children with anxiety disorders (47.1%; X2(1) = 5.11, p = 0.04; Figure 3a). Reaction times did not differ between children with and without anxiety disorders (F(1, 26) = 0.39, p = 0.54), or between the different stimuli (F(3, 24) = 0.20, p = 0.89). There was also no interaction between group and stimulus (F(3, 24) = 2.51, p = 0.08; Figure 3b).
Figure 3.
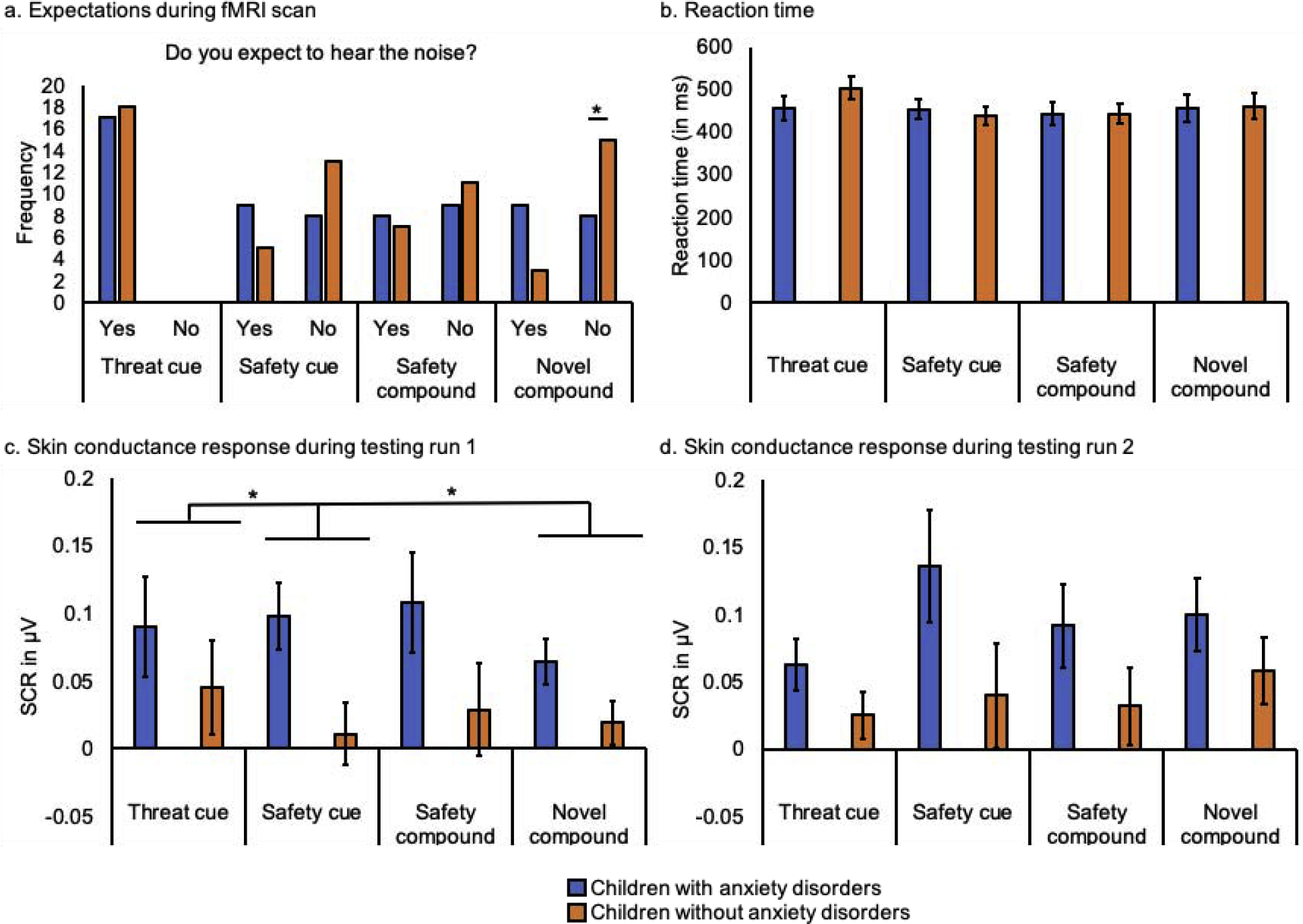
Behavioral and physiological responses to all stimuli during the testing phase of the conditioned inhibition task in children with and without anxiety disorders: (a) expectancy ratings after the testing phase during the fMRI scan, (b) reaction time, and skin conductance response (SCR) during (c) the first testing run and (d) the second testing run. Error bars display +/− 1 standard error.
3.2.2. Skin conductance response.
Children with anxiety disorders showed a higher SCR to all stimuli relative to children without anxiety disorders (F(1, 30) = 4.54, p = 0.04). SCR did not differ between the stimuli (F(3, 28) = 2.39, p = 0.09) and there was no interaction between group and stimulus (F(3, 28) = 0.74, p = 0.54), or between group and timing (F(1, 30) = 0.14, p = 0.71). A significant interaction between stimulus and timing (F(3, 28) = 4.45, p = 0.01) was explained by an effect of stimulus during the first testing run (F(3, 28) = 3.48, p = 0.03; Figure 3c), while there was no effect of stimulus in the second testing run (F(3, 28) = 2.84, p = 0.06; Figure 3d). Specifically, during the first testing run, children with and without anxiety disorders showed increased SCR to the threat cue compared to the safety cue (F(1, 30) = 5.09, p = 0.03) and compared to the novel compound (F(1, 30) = 4.68, p = 0.04).
3.2.3. ROI results.
ROI analyses of the testing phase focused on the difference between the safety compound and the novel compound. Across all children, there were no differences in activity between the safety and novel compounds in the amygdala, dACC, hippocampus, or vmPFC during the first or second testing run (all ts < 1.84, all ps > 0.07). There were no significant effects of group for activity in the amygdala, dACC, hippocampus, or left vmPFC during the first or second testing run (Fs < 3.06, ps > 0.09). During the first testing run, children with anxiety disorders showed more activity in the right vmPFC in response to the safety compound compared to the novel compound, whereas children without anxiety disorders showed more activity in the right vmPFC in response to the novel compound compared to the safety compound (F(1, 31) = 5.40, p = 0.03; Figure 4). There were no group differences in right vmPFC activity during the second testing run (F(1, 31) = 0.55, p = 0.46).
Figure 4.
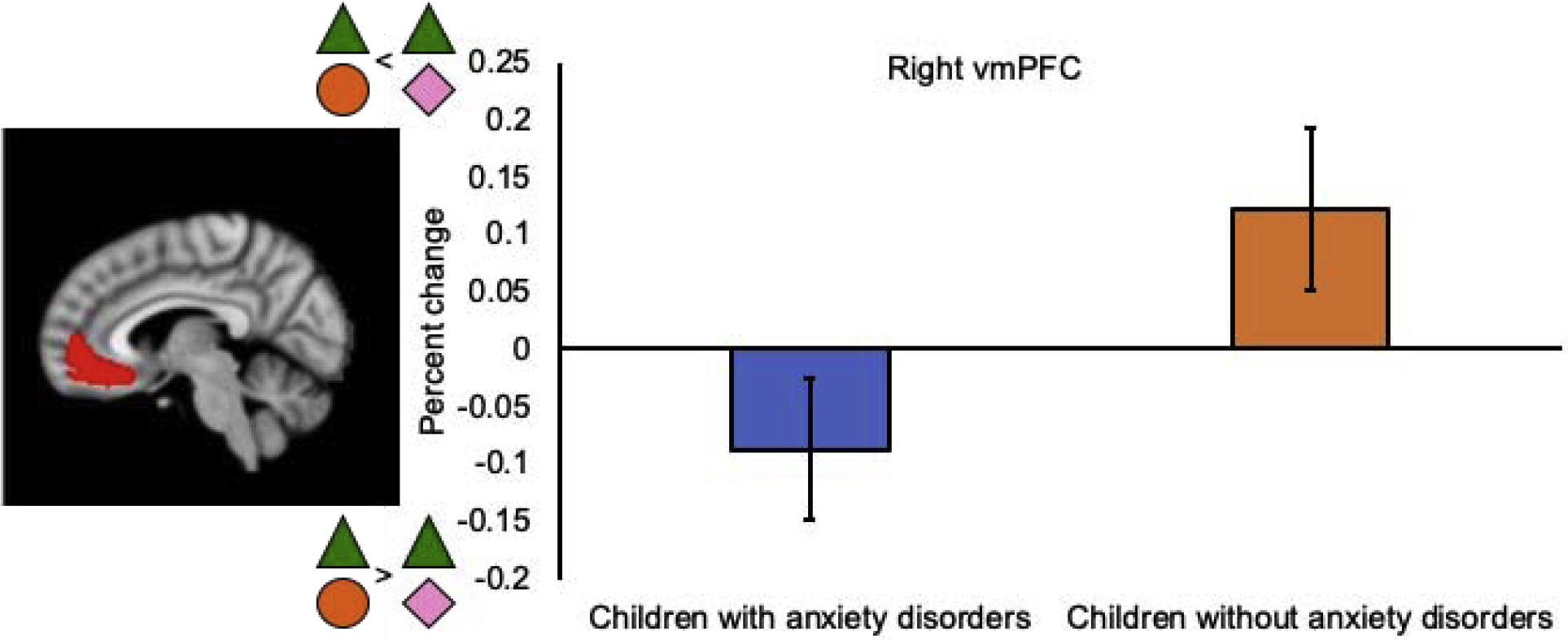
Effect of group in the right ventromedial prefrontal cortex (vmPFC) in the safety compound versus novel compound contrast during the first testing run. Error bars display +/− 1 standard error.
3.2.4. Whole-brain results.
There were no significant effects at the cluster-forming threshold of Z > 3.1. Overall, brain activity in response to the safety and novel compounds did not differ across all children during the first or second testing run. There were also no differences between children with and without anxiety disorders.
3.3. Extinction and reversal phases
There were no differences between children with and without anxiety disorders in expectancies, reaction time, SCR, or brain activity (neither ROI nor whole-brain effects) during the extinction phase (see supplementary material C for more details). There were also no differences between children with and without anxiety disorders in expectancies, reaction time, or brain activity (neither ROI nor whole-brain effects) during the reversal phase. There was an effect of group on SCR during the reversal phase, such that children with anxiety disorders showed a higher SCR to all stimuli relative to children without anxiety disorders (F(1, 30) = 4.48, p = 0.04).
4. Discussion
The goal of the current pilot study was to compare behavioral, physiological, and neural correlates of conditioned inhibition between children with and without anxiety disorders. Compared to children without anxiety disorders, children with anxiety disorders showed differential activity in the right vmPFC during an early phase of conditioned inhibition. Children with anxiety disorders also showed increased SCR to all stimuli during the testing phase, and they also more often expected to hear an aversive sound during a novel condition than children without anxiety disorders. These findings lay the groundwork for larger studies designed to examine more comprehensively how conditioned inhibition relates to pediatric anxiety.
Here we found preliminary evidence that neural processes during conditioned inhibition might differ between children with and without anxiety disorders. Specifically, children with anxiety disorders showed more activity in response to the safety compound compared to the novel compound in the right vmPFC during the first testing run. In contrast, children without anxiety disorders showed more activity in the right vmPFC in response to the novel compound compared to the safety compound. Previous studies have shown that the vmPFC plays an important role in threat and safety learning in anxious youth [35, 37, 63]. The current findings regarding vmPFC activity could suggest that conditioned inhibition is supported through different neural mechanisms in children with, relative to children without, anxiety disorders. Contrary to our hypotheses, children with and without anxiety disorders did not show differential activation in the amygdala, dACC, or hippocampus during conditioned inhibition, possibly due to the small sample size. Interactions between the dACC and hippocampus might be especially important for conditioned inhibition, as was recently shown in adults [26], and should be further investigated in larger studies of conditioned inhibition in clinically anxious youth.
The process of conditioned inhibition may be relevant for optimizing treatment of pediatric anxiety disorders. Although CBT is the first-line treatment for children with anxiety disorders, many children continue to meet criteria for an anxiety disorder following CBT [1, 2]. A translational approach to studying the mechanisms underlying fear reduction might provide information that could be used to improve treatment. Animal [5, 6] and human [26] studies have shown that conditioned inhibition effectively reduces fear responding. Here we found preliminary evidence that the effects of conditioned inhibition might differ in children with anxiety disorders. Much remains unknown about how the process of incorporating information about safety via conditioned inhibition in the laboratory compares to the use of safety cues in the real world. Nevertheless, this research may inform ongoing discussions about the use of safety cues in CBT. A cautious approach is required for incorporating safety cues into CBT, since safety behaviors have a role in maintaining anxiety disorders [64, 65]. However, the use of safety cues may nonetheless have practical value for CBT. For example, judicious use of safety cues may reduce aversiveness, facilitate adherence, and/or improve outcomes in youth for whom traditional CBT is not as effective. Indeed, systematic incorporation of safety cues into the beginning and most challenging parts of treatment has shown positive effects in adults [66]. Importantly, there are likely specific conditions under which safety cues may be more likely to facilitate versus interfere with symptom reduction. For example, evidence suggests that ‘restorative’ safety cues that allow for full confrontation with the core fear may have positive effects on exposure outcomes, whereas ‘preventive’ safety cues that hinder full confrontation may have negative effects on exposure outcomes [67]. Future research will be important to investigate how findings from studies of conditioned inhibition in children could inform treatment approaches for pediatric anxiety disorders [68].
During fear acquisition, children with and without anxiety disorders did not differ in their behavioral and physiological responses to threat and safety cues. Moreover, children showed a higher SCR to the safety cue, instead of to the threat cue, during fear acquisition. Given the pediatric sample, it is important to consider whether children fully understood the task. For example, when asked following acquisition during scan, many children (both with and without anxiety disorders) reported that they expected to hear the aversive sound with the safety cue but not the threat cue. However, a large proportion of children did not respond to the question about their expectations following the acquisition phase. Because children were instructed to respond via a button press and not verbally, we do not have additional information about missing responses. It is possible that we allocated insufficient time for responding, that children were not prepared for the question about their expectation, or that children did not understand the question or task itself. This will be important to consider in future task design: better pre-scanning training may be needed to teach children how to use the button box to respond to the in-scanner questions, or children may need a longer window to respond (in this study children were given 4 seconds to respond). Importantly, during the testing phase, all children reported that they expected to hear the aversive sound with the threat cue, and children showed increased SCR to the threat cue compared to the safety cue during the first testing run. In addition, after the MRI scan, children were asked again if they expected to hear the aversive sound in the beginning of the task, and most children reported then that they expected to hear the aversive sound with the threat cue and not with the safety cue. These findings suggest that children did indeed understand the difference between the threat and safety cues and that a lack of understanding is unlikely to account for the lack of differential behavioral and physiological responses to threat and safety cues during fear acquisition.
An alternative explanation for the lack of differential behavioral and physiological responses to threat and safety cues during acquisition might be that the UCS (i.e., aversive sound) was not aversive enough to elicit a robust fear response. This could be related to additional noise from the MRI scanner, or because children were already experiencing nervousness in the scan environment. It is also possible that habituation occurred relatively quickly (see Supplementary Figure A1), consistent with the effect of conditioned inhibition in the right vmPFC and differential SCR to the threat versus safety cue being specific to the first testing run. Future research should therefore investigate what the most effective UCS is in the MRI scanner for children. Shocks cannot be used due to ethical reasons, but an aversive sound could be paired with another aversive stimulus [70], such as a scary picture, to elicit a stronger fear response. Other options would be to explore using a higher reinforcement rate, or to ask children to rate the level of aversiveness of the UCS first and then calibrate the intensity of the UCS on these individual ratings. Selecting a UCS that is appropriate for use with children is an ongoing challenge in research on threat learning and pediatric anxiety disorders [27, 71], and methodological refinements will be important for identifying a UCS that is aversive enough to elicit a robust fear response but also not so upsetting that it produces high rates of attrition and discomfort.
This was the first study on conditioned inhibition in children with and without anxiety disorders, and several limitations should be taken into account. First, this was an exploratory study, so the sample size was small. Second, it is unclear if all children fully understood the task, especially the more complex parts (e.g., the safety and novel compounds). Moreover, expectancy ratings after the acquisition phase were missing for many children (57.1%). Future studies should ensure that children understand the task through more extensive training and pre-scanning testing of children’s understanding. Moreover, the chance of missing data could be reduced through pre-scanning training and longer response windows. Building upon the literature on instructed fear and extinction [72–74], another possibility would be to explicitly instruct children about the contingencies for the compound stimuli (e.g., that the safety compound will never be paired with the aversive noise). However, this approach would shift the focus of the task to fear expression instead of fear learning (please see Lonsdorf, Menz, Andreatta, Fullana, Golkar, Haaker, Heitland, Hermann, Kuhn, Kruse, Drexler, Meulders, Nees, Pittig, Richter, Romer, Shiban, Schmitz, Straube, Vervliet, Wendt, Baas and Merz [13] for an extensive review). Alternatively, it may be useful to explicitly point out to children that the compound stimuli are made up of the two individual shapes, without providing the information that the compound stimuli will never be paired with the aversive noise. Another option would be to increase the number of trials for the compound stimuli to provide a more extended opportunity for learning. Third, children with anxiety disorders had on average a higher IQ than children without anxiety disorders. We could not test for group differences in race, highest parental education, and household gross income due to the small sample size and missing data. Future studies should test for replication of these findings in a larger sample with groups that are matched on variables such as IQ and demographic variables. Fourth, ten children were excluded because they discontinued the task and did not have distortion correction scans. Although this exclusion was necessary because data with and without distortion correction were different, it limited the sample size. Furthermore, seven children who aborted the task only had data for two fMRI runs (i.e., acquisition and first testing run) and three children who aborted the task only had data during acquisition. This provides important information about the challenges of conducting threat learning studies in the scan environment with youth. Importantly, the present findings will need to be replicated in more robust samples and following methodological refinements to optimize the conditioned inhibition paradigm for use with children. Despite the preliminary nature of these results, they may provide important insight to help guide future research on conditioned inhibition during development, particularly among children with anxiety disorders.
Taken together, we found that children with anxiety disorders displayed differential activity in the vmPFC during conditioned inhibition. These findings could suggest that children with anxiety disorders incorporate information about learned safety differently at the neural level, with potential implications for the efficacy of conditioned inhibition for reducing fear. Future work with larger samples and methodological refinements might confirm altered conditioned inhibition in pediatric anxiety disorders and provide targets for CBT-resistant anxiety.
Supplementary Material
Highlights.
Cross-species research suggests that conditioned inhibition reduces fear responding.
This is the first study of conditioned inhibition in pediatric anxiety.
Children with anxiety disorders showed differential activity in vmPFC.
Future research should further examine conditioned inhibition in pediatric anxiety.
Acknowledgements
Declarations of interest: None.
This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (ZIA-MH-002782 and NCT00018057 for D.S. Pine), National Institutes of Health Director’s Early Independence Award (DP5OD021370 for. D.G. Gee), Brain & Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression; NARSAD) Young Investigator Award (for D.G. Gee), Jacobs Foundation Early Career Research Fellowship (for D.G. Gee), and National Science Foundation Graduate Research Fellowship Program Award (DGE1122492 for P.O).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CBT = cognitive-behavioral therapy; SCR = skin conductance response; UCS = unconditioned stimulus; dACC = dorsal anterior cingulate cortex; vmPFC = ventromedial prefrontal cortex; PTSD = posttraumatic stress disorder; CGI = Clinical Global Impression; MRI = magnetic resonance imaging; fMRI = functional magnetic resonance imaging; SCARED = Screen for Child Anxiety Related Disorders; STAI = Spielberger State-Trait Anxiety Inventory; SC = skin conductance; BOLD = blood oxygen level-dependent; TR = repetition time; FOV = field of view; FSL = Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library; ICA-AROMA = ICA-based strategy for automatic removal of motion artifacts; FD = framewise displacement; ROI = region of interest; FWER = family-wise error rate
A single-group paired t-test was conducted within participants who had distortion correction scans to compare results with and without including the distortion correction scans in the individual-level analysis. Several differences were found, suggesting there was some distortion in the scans that needed to be corrected. Thus, we included only participants with distortion correction scans.
We re-ran the analysis without the nuisance regressors in the individual-level analysis. The effects of group remained largely the same, except for one finding: Statistics for the group difference in the right vmPFC ROI during the first testing run were F(1, 31) = 4.13, p=0.051.
While mean FD did not relate to the majority of metrics of SCR and RT across all conditions and phases, mean FD was related to SCR to the threat cue (r = 0.43, p = 0.01), safety cue (r = 0.48, p = 0.01), and novel compound (r = 0.49, p = 0.003) during the first testing run, to SCR to the threat cue (r = 0.63, p < 0.001) during the extinction phase, and to reaction time to the threat cue during acquisition (r = −0.36, p = 0.04) and the first testing run (r = −0.34, p = 0.047).
References
- [1].Hudson JL, Rapee RM, Lyneham HJ, McLellan LF, Wuthrich VM, Schniering CA, Comparing outcomes for children with different anxiety disorders following cognitive behavioural therapy, Behaviour Research and Therapy 72 (2015) 30–37. [DOI] [PubMed] [Google Scholar]
- [2].Creswell C, Waite P, Hudson J, Practitioner Review: Anxiety disorders in children and young people - assessment and treatment, Journal of Child Psychology and Psychiatry (2020). [DOI] [PubMed] [Google Scholar]
- [3].Craske MG, Hermans D, Vervliet B, State-of-the-art and future directions for extinction as a translational model for fear and anxiety, Philos. Trans. R. Soc. B-Biol. Sci 373(1742) (2018) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Milad MR, Quirk GJ, Fear extinction as a model for translational neuroscience: Ten years of progress, in: Fiske ST, Schacter DL, Taylor SE (Eds.), Annual Review of Psychology, Vol 632012, pp. 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Myers KM, Davis M, AX+, BX− discrimination learning in the fear-potentiated startle paradigm: Possible relevance to inhibitory fear learning in extinction, Learn. Mem 11(4) (2004) 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kazama AM, Schauder KB, McKinnon M, Bachevalier J, Davis M, A novel AX+/BX− paradigm to assess fear learning and safety-signal processing with repeated-measure designs, J. Neurosci. Methods 214(2) (2013) 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui LH, Benjet C, Georgiades K, Swendsen J, Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A), J. Am. Acad. Child Adolesc. Psychiatr 49(10) (2010) 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Copeland WE, Angold A, Shanahan L, Costello EJ, Longitudinal patterns of anxiety from childhood to adulthood: The Great Smoky Mountains Study, J. Am. Acad. Child Adolesc. Psychiatr 53(1) (2014) 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Costello EJ, Egger HL, Angold A, The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and comorbidity, Child and Adolescent Psychiatric Clinics of North America 14(4) (2005) 631–648. [DOI] [PubMed] [Google Scholar]
- [10].Ezpeleta L, Keeler G, Erkanli A, Costello EJ, Angold A, Epidemiology of psychiatric disability in childhood and adolescence, J. Child Psychol. Psychiatry Allied Discip 42(7) (2001) 901–914. [DOI] [PubMed] [Google Scholar]
- [11].Essau CA, Conradt J, Petermann F, Frequency, comorbidity, and psychosocial impairment of anxiety disorders in German adolescents, J. Anxiety Disord 14(3) (2000) 263–279. [DOI] [PubMed] [Google Scholar]
- [12].Kessler RC, Berglund P, Demler O, Jin R, Walters EE, Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication, Archives of General Psychiatry 62(6) (2005) 593–602. [DOI] [PubMed] [Google Scholar]
- [13].Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, Heitland I, Hermann A, Kuhn M, Kruse O, Drexler SM, Meulders A, Nees F, Pittig A, Richter J, Romer S, Shiban Y, Schmitz A, Straube B, Vervliet B, Wendt J, Baas JMP, Merz CJ, Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear, Neurosci. Biobehav. Rev 77 (2017) 247–285. [DOI] [PubMed] [Google Scholar]
- [14].Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, Radua J, Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies, Molecular Psychiatry 21(4) (2016) 500–508. [DOI] [PubMed] [Google Scholar]
- [15].Etkin A, Wager TD, Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia, American Journal of Psychiatry 164(10) (2007) 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, Baas JMP, Updated meta-analysis of classical fear conditioning in the anxiety disorders, Depress. Anxiety 32(4) (2015) 239–253. [DOI] [PubMed] [Google Scholar]
- [17].Dvir M, Horovitz O, Aderka IM, Shechner T, Fear conditioning and extinction in anxious and non-anxious youth: A meta-analysis, Behaviour Research and Therapy 120 (2019) 7. [DOI] [PubMed] [Google Scholar]
- [18].Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, Radua J, Harrison BJ, Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants, Neurosci. Biobehav. Rev 88 (2018) 16–25. [DOI] [PubMed] [Google Scholar]
- [19].Phelps EA, Delgado MR, Nearing KI, LeDoux JE, Extinction learning in humans: Role of the amygdala and vmPFC, Neuron 43(6) (2004) 897–905. [DOI] [PubMed] [Google Scholar]
- [20].Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B, Maximizing exposure therapy: An inhibitory learning approach, Behaviour Research and Therapy 58 (2014) 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, Sangha S, Inhibition of fear by learned safety signals: A mini-symposium review, J. Neurosci 32(41) (2012) 14118–14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Woon EP, Seibert TA, Urbanczyk PJ, Ng KH, Sangha S, Differential effects of prior stress on conditioned inhibition of fear and fear extinction, Behav. Brain Res 381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meyer HC, Gerhard DM, Amelio PA, Lee FS, Pre-adolescent stress disrupts adult, but not adolescent, safety learning, Behav. Brain Res (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ, Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm, Biological Psychiatry 57(12) (2005) 1559–1564. [DOI] [PubMed] [Google Scholar]
- [25].Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ, Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity, Psychiatry Research 167(1–2) (2009) 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li AF, Yang RR, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, Gee DG, Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans, Proceedings of the National Academy of Sciences of the United States of America 116(52) (2019) 26970–26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shechner T, Hong M, Britton JC, Pine DS, Fox NA, Fear conditioning and extinction across development: Evidence from human studies and animal models, Biol. Psychol 100 (2014) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS, Distinct neural signatures of threat learning in adolescents and adults, Proceedings of the National Academy of Sciences of the United States of America 108(11) (2011) 4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Waters AM, Theresiana C, Neumann DL, Craske MG, Developmental differences in aversive conditioning, extinction, and reinstatement: A study with children, adolescents, and adults, Journal of Experimental Child Psychology 159 (2017) 263–278. [DOI] [PubMed] [Google Scholar]
- [30].Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry, J. Neurosci 33(10) (2013) 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Casey BJ, Glatt CE, Lee FS, Treating the developing versus developed brain: Translating preclinical mouse and human studies, Neuron 86(6) (2015) 1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing DQ, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS, Altered fear learning across development in both mouse and human, Proceedings of the National Academy of Sciences of the United States of America 109(40) (2012) 16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morriss J, Christakou A, van Reekum CM, Multimodal evidence for delayed threat extinction learning in adolescence and young adulthood, Sci Rep 9 (2019) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gold AL, Abend R, Britton JC, Behrens B, Farber MJ, Ronkin EG, Chen G, Leibenluft E, Pine DS, Age differences in the neural correlates of anxiety disorders: An fMRI study of response to learned threat, American Journal of Psychiatry 177(5) (2020) 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, Ernst M, Nelson EE, Leibenluft E, Shechner T, Pine DS, Response to Learned Threat: An fMRI Study in Adolescent and Adult Anxiety, American Journal of Psychiatry 170(10) (2013) 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, Dynamic mapping of human cortical development during childhood through early adulthood, Proceedings of the National Academy of Sciences of the United States of America 101(21) (2004) 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haddad ADM, Bilderbeck A, James AC, Lau JYF, Fear responses to safety cues in anxious adolescents: Preliminary evidence for atypical age-associated trajectories of functional neural circuits, Journal of Psychiatric Research 68 (2015) 301–308. [DOI] [PubMed] [Google Scholar]
- [38].Chauret M, Suffren S, Pine DS, Nassim M, Saint-Amour D, Maheu FS, Fear conditioning and extinction in anxious youth, offspring at-risk for anxiety and healthy comparisons: An fMRI study, Biol. Psychol 148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chan KH, Jarrard LE, Davidson TL, The effects of selective ibotenate lesions of the hippocampus on conditioned inhibition and extinction, Cognitive Affective & Behavioral Neuroscience 3(2) (2003) 111–119. [DOI] [PubMed] [Google Scholar]
- [40].Heldt SA, Coover GD, Falls WA, Posttraining but not pretraining lesions of the hippocampus interfere with feature-negative discrimination of fear-potentiated startle, Hippocampus 12(6) (2002) 774–786. [DOI] [PubMed] [Google Scholar]
- [41].McDonald RJ, Balog RJ, Lee JQ, Stuart EE, Carrels BB, Hong NS, Rats with ventral hippocampal damage are impaired at various forms of learning including conditioned inhibition, spatial navigation, and discriminative fear conditioning to similar contexts, Behav. Brain Res 351 (2018) 138–151. [DOI] [PubMed] [Google Scholar]
- [42].Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandell ER, An animal model of a behavioral intervention for depression, Neuron 60(1) (2008) 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, Schedule for Affective Disorders and Schizophrenia for School-Age Children Present and Lifetime version (K-SADS-PL): Initial reliability and validity data, J. Am. Acad. Child Adolesc. Psychiatr 36(7) (1997) 980–988. [DOI] [PubMed] [Google Scholar]
- [44].Guy W, The clinical global impression scale, The ECDEU assessment manual for psychopharmacology, U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research, Rockville, MD, 1976, pp. 218–222. [Google Scholar]
- [45].Wechsler D, Wechsler Abbreviated Scale of Intelligence, The Psychological Corporation, San Antonio, TX, 1999. [Google Scholar]
- [46].Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM, The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics, J. Am. Acad. Child Adolesc. Psychiatr 36(4) (1997) 545–553. [DOI] [PubMed] [Google Scholar]
- [47].Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA, Manual for the State-Trait Anxiety Inventory, Consulting Psychologists Press, Palo Alto, CA, 1983. [Google Scholar]
- [48].Behrens B, Swetlitz C, Pine DS, Pagliaccio D, The Screen for Child Anxiety Related Emotional Disorders (SCARED): Informant discrepancy, measurement Iivariance, and test-retest reliability, Child Psychiatry & Human Development 50(3) (2019) 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abend R, Bajaj MA, Coppersmith DDL, Kircanski K, Haller SPW, Cardinale EM, Salum GA, Wiers RW, Salemink E, Pettit JW, Perez-Edgar K, Lebowitz ER, Silverman WK, Bar-Haim Y, Brotman MA, Leibenluft E, Fried EI, Pine DS, A computational network perspective on pediatric anxiety symptoms, Psychological Medicine (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Michalska KJ, Feldman JS, Ivie EJ, Shechner T, Sequeira S, Averbeck B, Degnan KA, Chronis-Tuscano A, Leibenluft E, Fox NA, Pine DS, Early-childhood social reticence predicts SCR-BOLD coupling during fear extinction recall in preadolescent youth, Developmental Cognitive Neuroscience 39(100605) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang YY, De Stefano N, Brady JM, Matthews PM, Advances in functional and structural MR image analysis and implementation as FSL, Neuroimage 23 (2004) S208–S219. [DOI] [PubMed] [Google Scholar]
- [52].Smith SM, Fast robust automated brain extraction, Hum. Brain Mapp 17(3) (2002) 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jenkinson M, Bannister P, Brady M, Smith S, Improved optimization for the robust and accurate linear registration and motion correction of brain images, Neuroimage 17(2) (2002) 825–841. [DOI] [PubMed] [Google Scholar]
- [54].Andersson JLR, Skare S, Ashburner J, How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging, Neuroimage 20(2) (2003) 870–888. [DOI] [PubMed] [Google Scholar]
- [55].Woolrich MW, Ripley BD, Brady M, Smith SM, Temporal autocorrelation in univariate linear modeling of FMRI data, Neuroimage 14(6) (2001) 1370–1386. [DOI] [PubMed] [Google Scholar]
- [56].Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF, ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data, Neuroimage 112 (2015) 267–277. [DOI] [PubMed] [Google Scholar]
- [57].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion, Neuroimage 59(3) (2012) 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ, Decreased volume of left and total anterior insular lobule in schizophrenia, Schizophrenia Research 83(2–3) (2006) 155–171. [DOI] [PubMed] [Google Scholar]
- [59].Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ, The integration of negative affect, pain and cognitive control in the cingulate cortex, Nature Reviews Neuroscience 12(3) (2011) 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hindy NC, Turk-Browne NB, Action-based learning of multistate objects in the medial temporal lobe, Cereb. Cortex 26(5) (2016) 1853–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mackey S, Petrides M, Architecture and morphology of the human ventromedial prefrontal cortex, Eur. J. Neurosci 40(5) (2014) 2777–2796. [DOI] [PubMed] [Google Scholar]
- [62].Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM, Multilevel linear modelling for FMRI group analysis using Bayesian inference, Neuroimage 21(4) (2004) 1732–1747. [DOI] [PubMed] [Google Scholar]
- [63].Ganella DE, Drummond KD, Ganella EP, Whittle S, Kim JH, Extinction of conditioned fear in adolescents and adults: A human fMRI study, Front. Hum. Neurosci 11 (2018) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rapee RM, Heimberg RG, A cognitive-behavioral model of anxiety in social phobia, Behaviour Research and Therapy 35(8) (1997) 741–756. [DOI] [PubMed] [Google Scholar]
- [65].Clark DM, Wells A, A cognitive model of social phobia, in: Heimberg R, Liebowitz M, Hope DA, Schneier FR (Eds.), Social phobia: Diagnosis, assessment and treatment, Guilford Press, New York, NY, 1995, pp. 69–93. [Google Scholar]
- [66].Blakey SM, Abramowitz JS, The effects of safety behaviors during exposure therapy for anxiety: Critical analysis from an inhibitory learning perspective, Clinical Psychology Review 49 (2016) 1–15. [DOI] [PubMed] [Google Scholar]
- [67].Goetz AR, Davine TP, Siwiec SG, Lee HJ, The functional value of preventive and restorative safety behaviors: A systematic review of the literature, Clinical Psychology Review 44 (2016) 112–124. [DOI] [PubMed] [Google Scholar]
- [68].Odriozola P, Gee DG, Learning about safety: Conditioned inhibition as a novel approach to fear reduction targeting the developing brain, American Journal of Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Abend R, Gold AL, Britton JC, Michalska KJ, Shechner T, Sachs JF, Winkler AM, Leibenluft E, Averbeck BB, Pine DS, Anticipatory threat responding: Associations with anxiety, development, and brain structure, Biological Psychiatry 87(10) (2020) 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kredlow MA, Orr SP, Otto MW, Who is studied in de novo fear conditioning paradigms? An examination of demographic and stimulus characteristics predicting fear learning, Int. J. Psychophysiol 130 (2018) 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ryan KM, Zimmer-Gembeck MJ, Neumann DL, Waters AM, The need for standards in the design of differential fear conditioning and extinction experiments in youth: A systematic review and recommendations for research on anxiety, Behaviour Research and Therapy 112 (2019) 42–62. [DOI] [PubMed] [Google Scholar]
- [72].Atlas LY, Doll BB, Li J, Daw ND, Phelps EA, Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala, eLife 5 (2016) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Luck CC, Lipp OV, Instructed extinction in human fear conditioning: History, recent developments, and future directions, Australian Journal of Psychology 68(3) (2016) 209–227. [Google Scholar]
- [74].Javanbakht A, Duval ER, Cisneros ME, Taylor SF, Kessler D, Liberzon I, Instructed fear learning, extinction, and recall: additive effects of cognitive information on emotional learning of fear, Cognition & Emotion 31(5) (2017) 980–987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


