Summary
In 2008, the Radiation Therapy Oncology Group (RTOG) published an international collaborative atlas to define the clinical target volume (CTV) for pelvic radiation therapy in the postoperative treatment of endometrial and cervical cancer. This article updates the atlas using the last decade of knowledge on target definitions, expands the atlas to include the para-aortic region and inferior obturator region and removes all references to bony landmarks.
Purpose:
Accurate target definition is critical for the appropriate application of radiation therapy. In 2008, the Radiation Therapy Oncology Group (RTOG) published an international collaborative atlas to define the clinical target volume (CTV) for intensity modulated pelvic radiation therapy in the postoperative treatment of endometrial and cervical cancer. The current project is an updated consensus of CTV definitions, with removal of all references to bony landmarks and inclusion of the para-aortic and inferior obturator nodal regions.
Methods and Materials:
An international consensus guideline working group discussed modifications of the current atlas and areas of controversy. A document was prepared to assist in contouring definitions. A sample case abdominopelvic computed tomographic image was made available, on which experts contoured targets. Targets were analyzed for consistency of delineation using an expectation-maximization algorithm for simultaneous truth and performance level estimation with kappa statistics as a measure of agreement between observers.
Results:
Sixteen participants provided 13 sets of contours. Participants were asked to provide separate contours of the following areas: vaginal cuff, obturator, internal iliac, external iliac, presacral, common iliac, and para-aortic regions. There was substantial agreement for the common iliac region (sensitivity 0.71, specificity 0.981, kappa 0.64), moderate agreement in the external iliac, para-aortic, internal iliac and vaginal cuff regions (sensitivity 0.66, 0.74, 0.62, 0.59; specificity 0.989, 0.966, 0.986, 0.976; kappa 0.60, 0.58, 0.52, 0.47, respectively), and fair agreement in the presacral and obturator regions (sensitivity 0.55, 0.35; specificity 0.986, 0.988; kappa 0.36, 0.21, respectively). A 95% agreement contour was smoothed and a final contour atlas was produced according to consensus.
Conclusions:
Agreement among the participants was most consistent in the common iliac region and least in the presacral and obturator nodal regions. The consensus volumes formed the basis of the updated NRG/RTOG Oncology postoperative atlas. Continued patterns of recurrence research are encouraged to refine these volumes.
Introduction
Endometrial cancer and early stage cervical cancer are treated with surgery with or without adjuvant radiation therapy or chemotherapy.1–7 For endometrial cancer, adjuvant radiation therapy has been shown to decrease locoregional recurrence for patients with stage I disease with intermediate or high-risk features.1,3,4 Population-based studies suggest that adjuvant radiation therapy for patients with endometrial cancer can improve overall survival.8–10 For cervical cancer, adjuvant radiation therapy alone decreases locoregional recurrence for patients with intermediate risk factors; the addition of concurrent chemotherapy to adjuvant pelvic radiation therapy improves overall survival for patients with high-risk factors.6,7,11
In the era of computed tomography (CT) simulation, 3-dimensional conformal radiation therapy (3D-CRT) was used to deliver adjuvant radiation therapy, using target volume-based contouring with consideration of traditional bony landmarks to delineate the treatment volume. More recently, intensity modulated radiation therapy (IMRT) enables delivery of the prescribed dose to at-risk targets while limiting the dose to adjacent normal tissues. The feasibility of IMRT has been demonstrated with acceptable toxicity profiles for the treatment of gynecologic malignancies.12–14 The NRG/Radiation Therapy Oncology Group (RTOG) 1203 (TIME-C) trial showed that in the treatment of postoperative endometrial and cervical cancer patients IMRT reduces rates of acute and chronic bowel and urinary toxicity while improving quality of life compared with 3D-CRT.15,16
However, IMRT requires a more accurate understanding and delineation of the clinical target volume (CTV) than may be necessary for 3D-CRT. In 2008, the RTOG published their guidelines for CTV delineation in postoperative treatment of gynecologic malignancies. This document used bony landmarks as guides, did not include guidelines for paraaortic nodal volume delineation, and did not identify individual nodal groups in the contouring atlas.17 The addition of the para-aortic region was accomplished to expand the applicability of the guidelines to include patients who necessitate extended field radiation. The choice to include individual nodal groups was to quantify the areas that may be particularly difficult to define. The purpose of this study is to update the previously described consensus guidelines for the CTV delineation of postoperative endometrial and cervical cancer, which can be used in either the 3-dimensional (3D) or IMRT setting.
Methods and Materials
An international consortium of radiation oncologists was established to include members of the RTOG Gynecologic Working Group, international radiation oncologists with a special interest in gynecologic malignancies, a gynecologic oncologist, and a radiologist with specialized knowledge in gynecologic imaging. A sample postoperative case was chosen as the representative case, and the radiation oncologists were asked to provide separate nodal group and vaginal contours to represent the CTV for the following targets: vaginal CTV and the obturator, internal iliac, external iliac, presacral, common iliac, and para-aortic nodal regions. Anatomic variants are not uncommon, and clinical situations such as variable bladder and rectal filling require the physician to adapt these guidelines. The current analysis included nearly 2500 contours and to attempt to analyze all clinical scenarios was not practical. Although an internal target volume (ITV) is commonly used and recommended to account for motion of the vagina, parametria, and adjacent organs, an ITV consensus contour was determined to be beyond the scope of this atlas; however, its importance is addressed in the Discussion. Members from the same institution performed the contours together so that each institution was represented only once. Sixteen participants provided thirteen sets of contours using their own contouring software.
The contours were submitted as DICOM files to the Imaging and Radiation Oncology Core–St. Louis QA Center for analysis. An expectation-maximization algorithm for simultaneous truth and performance level estimation18 was used to estimate the underlying true contour for each structure and the sensitivity (fraction of “true” structure voxels included) and specificity (fraction of voxels outside the “true” structure excluded) of each delineation. For each structure, a 95% confidence interval (CI) contour was generated as a candidate for consensus. Kappa statistics were used to compare degree of agreement between contours.19 Conformity index using the mean-to-union ratio was calculated. An example of the overlayed contours and the 95% CI contour is shown in Fig. 1.
Fig. 1.
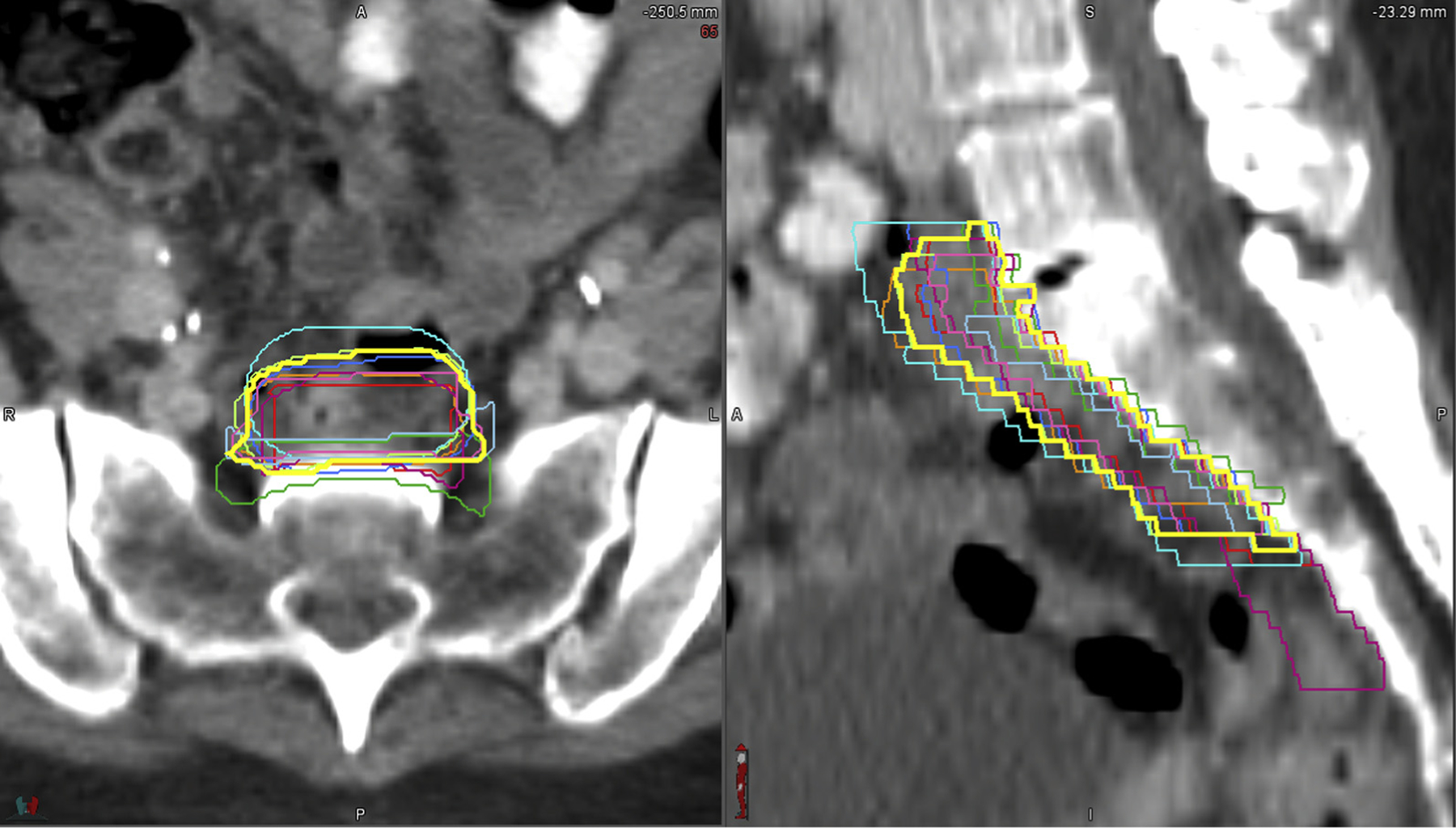
Individual contour overlay and the 95% confidence interval contour for the pre-sacral region.
The 95% CI contours were smoothed and slightly altered to correct any irregularities that resulted from their generation. These contours were then reviewed by the expert radiation oncologists using MIM software (MIM Software, Cleveland, OH). Comments were sent to the primary authors and addressed during 2 conference calls with real-time alterations made to the 95% CI contours until consensus was reached. The contouring atlas was generated in PDF format to include pertinent annotations that arose during conference call discussions. Every effort was made to provide an evidence based final product based on peer reviewed literature and supplemented by expert opinion. The final contours were again reviewed by the expert radiation oncologists, gynecologic oncologist, and radiologist until all contributing authors were satisfied. The final contouring atlas is available online at https://www.nrgoncology.org/ciro-gynecologic.
Results
Participants were asked to provide separate CTV contours of the following areas: vaginal cuff, obturator, internal iliac, external iliac, presacral, common iliac, and para-aortic. There was substantial agreement for the common iliac region (sensitivity 0.71, specificity 0.981, kappa 0.64). There was moderate agreement in the external iliac, para-aortic, internal iliac, and vaginal cuff regions (sensitivity 0.66, 0.74, 0.62, 0.59; specificity 0.989, 0.966, 0.986, 0.976; kappa 0.60, 0.58, 0.52, 0.47, respectively). The presacral and obturator regions had fair agreement (sensitivity 0.55, 0.35 specificity 0.986, 0.988; kappa 0.36, 0.21, respectively). See Table 1 for a summary of the results.
Table 1.
Results from contouring gynecologic postoperative case on computed tomography by physician experts
| Staple agreement |
|||||||
|---|---|---|---|---|---|---|---|
| Substantial |
Moderate |
Fair |
|||||
| Structure measure | Common iliac (10 contours) |
External iliac (10 contours) |
Para-aortic (11 contours) |
Internal iliac (9 contours) |
Vaginal cuff (12 contours) |
Pre-sacral (9 contours) |
Obturator (9 contours) |
| Sensitivity | 71% | 66% | 74% | 62% | 59% | 55% | 35% |
| Specificity | 98.1% | 98.9% | 96.6% | 98.6% | 97.6% | 98.6% | 98.8% |
| Volume, mean/min/max, cm3 (SD) | 104.4/64.6/151.3 (26.5) | 139.0/85.8/277.8 (54.1) | 119.4/78.9/178.2 (33.9) | 130.6/87.0/277.9 (61.8) | 91.8/29.7/180.6 (47.5) | 50.4/17.1/143.2 (37.4) | 55.3/13.8/133.0 (34.6) |
| STAPLE/Intersection/Union Volume, cm3 | 116.7/31.7/234.4 | 158.0/37.7/372.8 | 108.2/39.5/312.7 | 145.0/23.6/387.7 | 117.9/0.2/270.7 | 51.4/3.5/216.8 | 77.0/0/255.3 |
| Kappa | 0.64 | 0.60 | 0.58 | 0.52 | 0.47 | 0.36 | 0.21 |
| Conformity index (Mean volume/Union volume) | 0.445 | 0.372 | 0.382 | 0.337 | 0.339 | 0.232 | 0.217 |
Abbreviations: STAPLE = simultaneous truth and performance level estimation.
Volume delineation
Para-aortic nodal CTV
Coverage of the para-aortic lymph node chain should be included when there is pathologic or radiographic evidence of para-aortic lymph node involvement or enough of a risk of microscopic disease that the clinician feels the para-aortic region should be treated.
The para-aortic nodal CTV includes the lymph node groups at risk adjacent to the aorta and inferior vena cava (IVC): the paracaval, precaval, retrocaval, deep and superficial intercavo-aortic, para-aortic, preaortic, and retro-aortic nodes.20 Generally for cervical cancer, the superior border is at the level of the left renal vein, as there is low risk of nodal involvement above this level.21,22 However, if an individual has FDG avid nodes in the high para-aortic region, one might consider raising the superior border. For endometrial cancer, the superior border is slightly higher at 1 to 1.5 cm superior to the left renal vessels (Fig. 2a), although data on this superior extent are limited in the endometrial cancer setting. Individual anatomic variation can lead to the kidneys being in close proximity to the outlined volume. Evaluation of individual kidney function using, for example, a mercapto acetyl triglycine (MAG3) scan, could be considered in such cases. Although an intravenous (IV) contrast scan can help to locate the vessels, a noncontrast CT should be used for CTV delineation. The IV contrast can distend the IVC as (Fig. 2a–c), which can lead to excessive contouring. Figure 3 shows the difference in position of the IVC with similar CTV on a contrast scan (Figs. 3a, 3c) and non–contrast scan (Figs. 3b, 3d). The para-aortic nodal CTV encompasses the aorta, IVC and adjacent nodal regions however the para-aortic nodal CTV is not a uniform expansion off the vasculature (Fig. 3). This CTV should extend laterally from the aorta to the medial border of the left psoas muscle and include any visible small nodes in this region; this typically places the left lateral border 1 to 2 cm lateral to the aorta. On the right, the CTV margin is typically within 3 to 5 mm around the IVC. There is minimal evidence of nodal involvement to the right of the IVC and immediately anterior to the aorta and IVC where margins should be tighter. The space between the aorta and IVC should be straight (rather than concave), even if there is bowel nearby as the intercavo-aortic nodes are located in this space (Fig. 2c). The Keenan et al21 atlas uses similar margins, achieving coverage in the majority of nodal involvement in cervical cancer patients. However, there are limitations to the atlas’s methods, and the authors of this article agree that there is minimal evidence of nodal involvement to the right of the IVC, and a large expansion here can unnecessarily increase the dose to the right kidney.23 The para-aortic nodal CTV becomes the common iliac nodal CTV at the level of the aortic bifurcation.
Fig. 2.
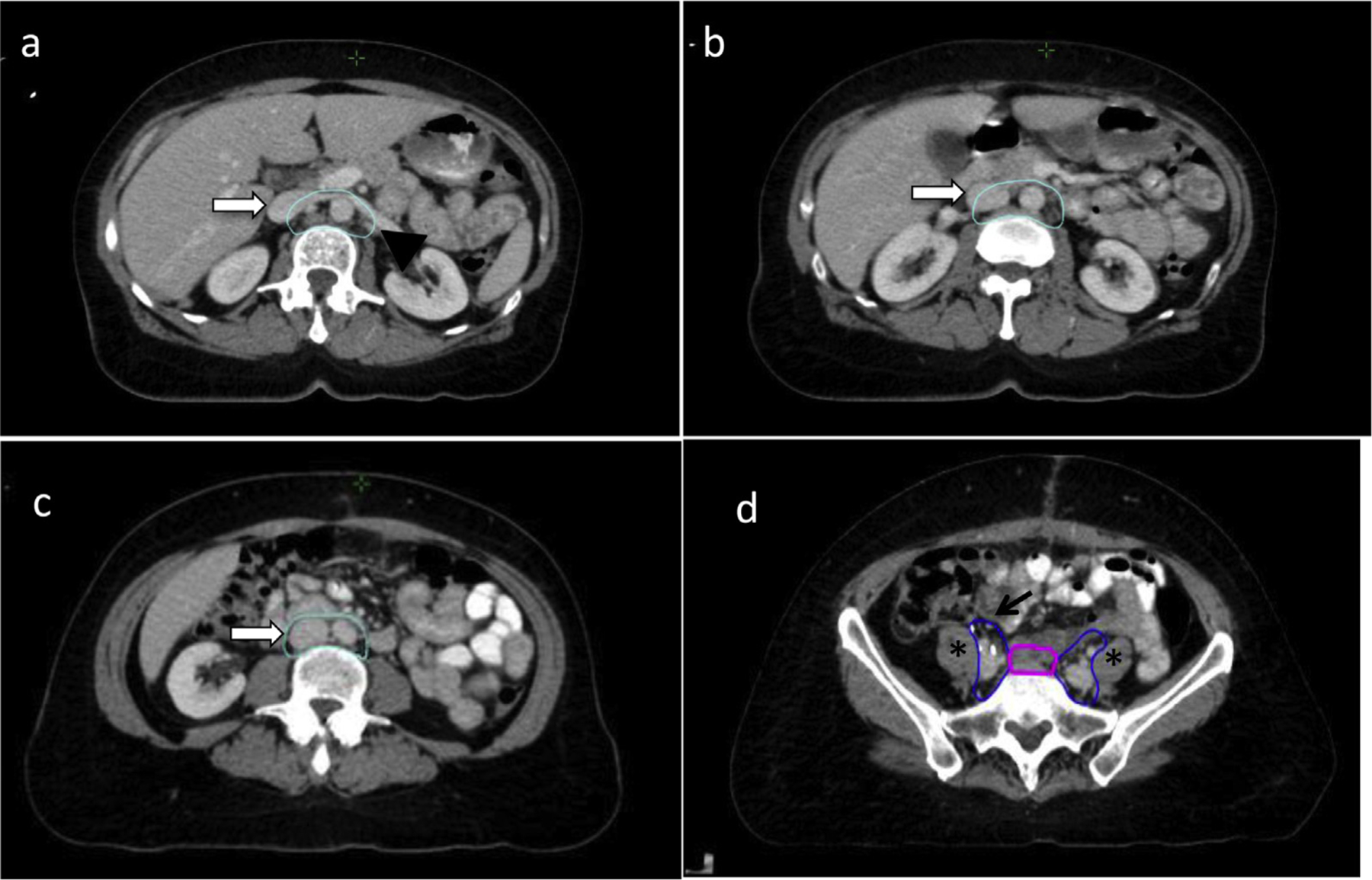
Para-aortic and common iliac nodal clinical target volumes (CTVs). (a) The superior portion of para-aortic nodal CTV (cyan) begins at or 1 to 1.5 cm above the left renal vessels. At this level, the inferior vena cava (IVC) is distended on the IV contrast scan (white arrow). The contour extends laterally abutting the psoas muscles (black arrowhead). (b) The distended IVC (white arrow) on the intravenous (IV) contrast scan is gradually included into the volume inferiorly. (c) There should not be a concavity to the contour in the space between the aorta and IVC in this region to assure coverage of the inter-aortocaval nodes. (d) The common iliac node CTV (blue) at the midportion of the chain should extend approximately 1 cm anterolateral to the common iliac artery (black arrow), along the iliopsoas muscle (asterisks). Also pictured is the presacral nodal CTV (magenta).
Fig. 3.
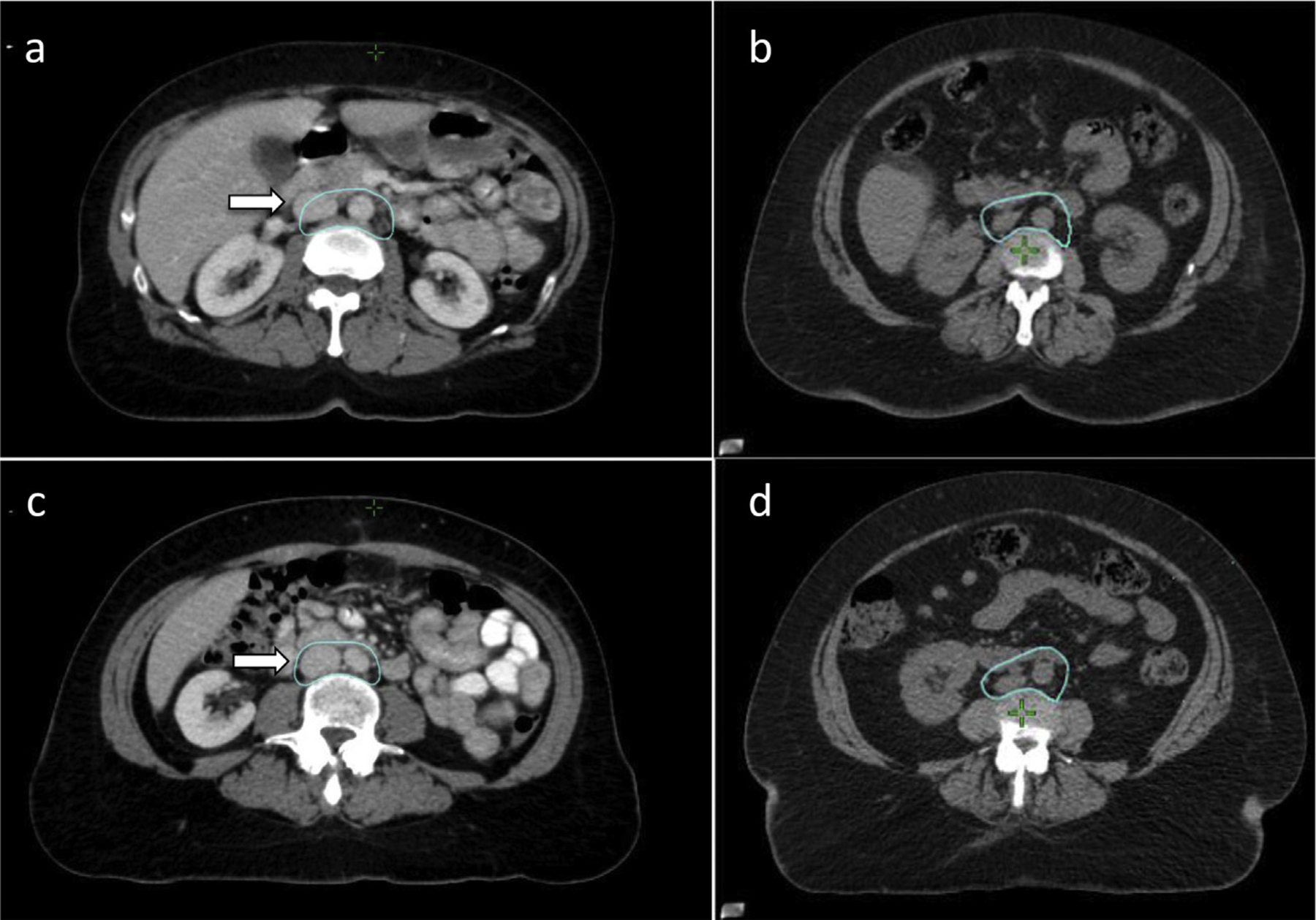
Para-aortic nodal clinical target volume (CTV) on intravenous (IV) contrast scan versus non–contrast scan. The superior para-aortic nodal CTVon (a) an IV contrast scan and (b) non–contrast scan. Inferior para-aortic nodal CTVon (c) an IV contrast scan and (d) a non–contrast scan. Note the difference in IVC distention on the contrast scan that requires a gradual inclusion of the IVC with margin compared with recommended expansions off the nondistended IVC and aorta on the contrast scan.
Common iliac nodal CTV
The CTV should continue with a 7-mm uniform margin surrounding the right and left common iliac artery and vein excluding bone and muscle. The contour should be extended to include any adjacent suspicious lymph nodes and any pertinent surgical clips as identified by the surgeon. To improve nodal coverage, the CTV margin should increase to 1 cm at the midpoint of the common iliac chain anterior to the vessels while abutting the iliopsoas laterally, as there can be lymph nodes that can extend to this region (Fig. 2d). The common iliac nodal CT extends inferiorly until the bifurcation of the common iliac artery.
Presacral nodal CTV
The presacral nodal CTV is a node-bearing strip of tissue anterior to the superior sacral bodies; it connects the right and left common iliac nodal CTV. The presacral lymph nodes should be included for all postoperative cervical cancer patients and in patients with endometrial cancer and cervical stromal invasion. The use of presacral radiation in other high-risk endometrial cancer patients is controversial, but 3 recent trials of intermediate or high-risk patients should help to clarify this topic. In PORTEC 3, the authors recommended inclusion of a 10-mm strip of tissue anterior to S1–S2 vertebral bodies, whereas in GOG 249 and GOG 258, the treatment of the presacral space was left to the discretion of the treating physician.24–26 In addition, the FIRES trial was a sentinel lymph node trial in clinically early stage endometrial cancer designed to test the sensitivity and negative predictive value of sentinel node mapping compared with complete dissection. Their results showed high sensitivity (97.2%) in detecting positive nodes, with only a 3% sentinel node metastasis rate in the presacral nodes. However, the exact risk factors for presacral nodal involvement in these patients are not known, including the degree of cervical involvement. Furthermore, in the FIRES study, 40% of all patients with a positive sentinel node had additional positive nodes on completion dissection, of which 20% of these were at a higher location, although exact location is not known.27 Given the limited data in the endometrial cancer setting, the authors suggest coverage of presacral nodes in cases with cervical stromal involvement and careful consideration of coverage in patients who have known lymph node metastases or high-risk factors for nodal involvement. If the presacral nodes are not included, then the common iliac nodal CTV will continue inferiorly until the bifurcations of the common iliac vessels, with no inclusion of this strip of tissue anterior to the sacrum.
The superior aspect of the presacral nodal CTV is contiguous with the deviation of the right and left common iliac nodal CTV. The presacral nodal CTV should be a relatively fixed strip of tissue measuring 1 to 1.5cm wide in a plane perpendicular to the face of the sacral bodies that starts superiorly at the bifurcation of the aorta into the common iliac vessels. When viewed in the axial plane, the amount of space that needs to be contoured can vary according to the shape of the sacrum. Coverage of the presacral space should always be confirmed by a review of the contour in the midline sagittal view. If bowel is present in this region, the CTV might extend a few millimeters into the bowel loops that might intermittently move into the presacral nodal CTV space (Fig. 4a). The inferior extent of the presacral nodal CTV is the first CT image in which the piriformis muscle becomes apparent (Fig. 4b).
Fig. 4.
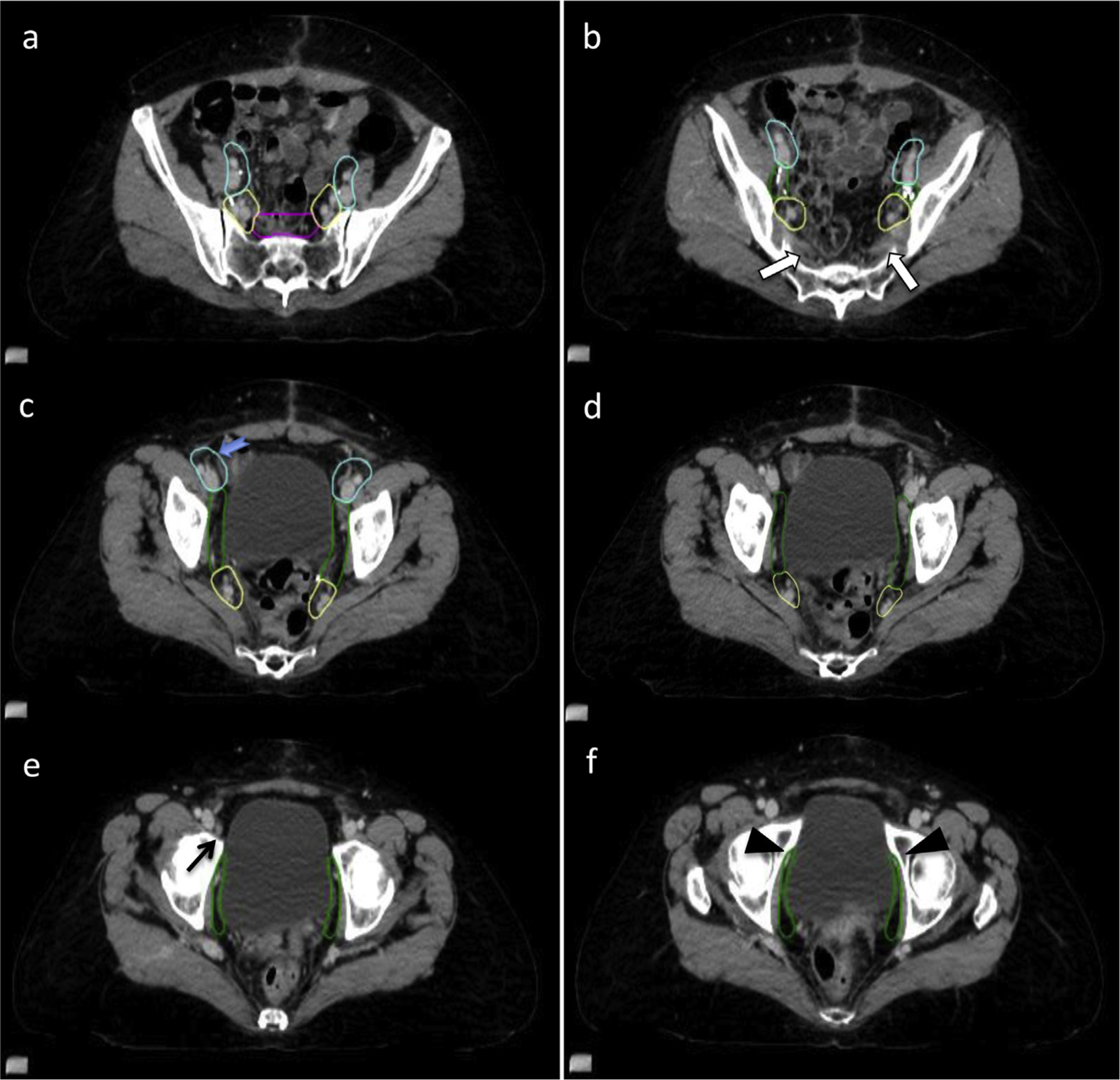
Presacral, internal and external iliac nodal clinical target volumes (CTVs). (a) The presacral nodal CTV (magenta) sits anterior to S1/S2 vertebral bodies and should be 1 to 1.5 cm wide, and it may encompass adjacent bowel if present, to account for motion of the bowel. (b) The insertion of the piriformis muscle (white arrows) on the sacrum marks the inferior extent of the presacral nodes. (c) The inferior extent of the external iliac nodal CTV (cyan) is seen either where the circumflex vessels originate from the external iliac vessels (blue arrow) or where external iliac vessels turn laterally to become the inguinofemoral vessels. (d) Similarly, the inferior extent of the internal iliac nodal CTV (yellow) should stop as the internal iliac vessels turn laterally to leave the pelvis. (e) Inferior to the external iliac CTV lays the circumflex node (black arrow), which is often enlarged, but it is rarely malignant, thus is not typically included. (f) The obturator vessels leave the pelvis through the obturator notch (black arrowheads), which marks the inferior extent of the obturator nodal CTV (green).
External iliac nodal CTV
The external iliac nodal CTV begins superiorly at the bifurcation of the common iliac vessels and should comprise a uniform 7-mm margin placed around the vessels not extending into bone or muscle and up to 10 mm anteriorly.28 The inferior aspect of the external iliac arteries is where the deep circumflex artery branches and where the external iliac vessels course laterally as they leave the pelvis to become the inguinofemoral vessels (Fig. 4c).
The circumflex iliac node is the most distal external iliac node, and it is often enlarged on imaging related to draining the lower extremity, although it is uncommonly involved with cancer.29 In a series of patients with cervical cancer, those who had negative pelvic nodes also had a negative circumflex node, and only 8% of patients with positive pelvic nodes had a positive circumflex node. Therefore, the circumflex node was deemed an appropriate caudal surgical landmark to limit an external iliac lymphadenectomy.30 This node can be seen in Figure 4e, and it is appropriately not included in the target CTV. However, the inclusion of this node in the target CTV should be individualized on the basis of the individual patient’s extent of more proximal external iliac nodal disease.
Internal Iliac Nodal CTV
The internal iliac nodal CTV begins superiorly at the bifurcation of the common iliac vessels with a uniform radial margin of 7 mm excluding bone and muscle. This volume courses distally until the vessels turn laterally before leaving the pelvis. The risk of nodal involvement inferior to this is believed to be low; therefore, the contour discontinues when the vessels turn laterally, which occurs before they truly leave the pelvis (Fig. 4d). Extending this volume inferiorly unnecessarily increases the dose to the rectum; thus, the group consensus was that the additional risk was not worth the little additional benefit to extending the contour further inferiorly.
Obturator nodal CTV
The obturator nodal CTV is a strip of space about 15 to 18 mm in diameter between the external and internal iliac nodal CTV contours, with the superior aspect starting at the bifurcation of the internal and external iliac vessels, not extending into bone, muscle, or bladder.28 The inferior extent of the contour discontinues when the obturator vessels leave the pelvis through the obturator foramen, which lies lateral to the obturator internus muscle (Fig. 4f). The use of an obturator nodal ITV should be considered, accounting for changes in bladder filling as described in Fig. 5 and in the Discussion. If an obturator nodal ITV is not used, then an appropriate planning treatment volume (PTV) margin extending into the bladder is necessary as discussed later.
Fig. 5.
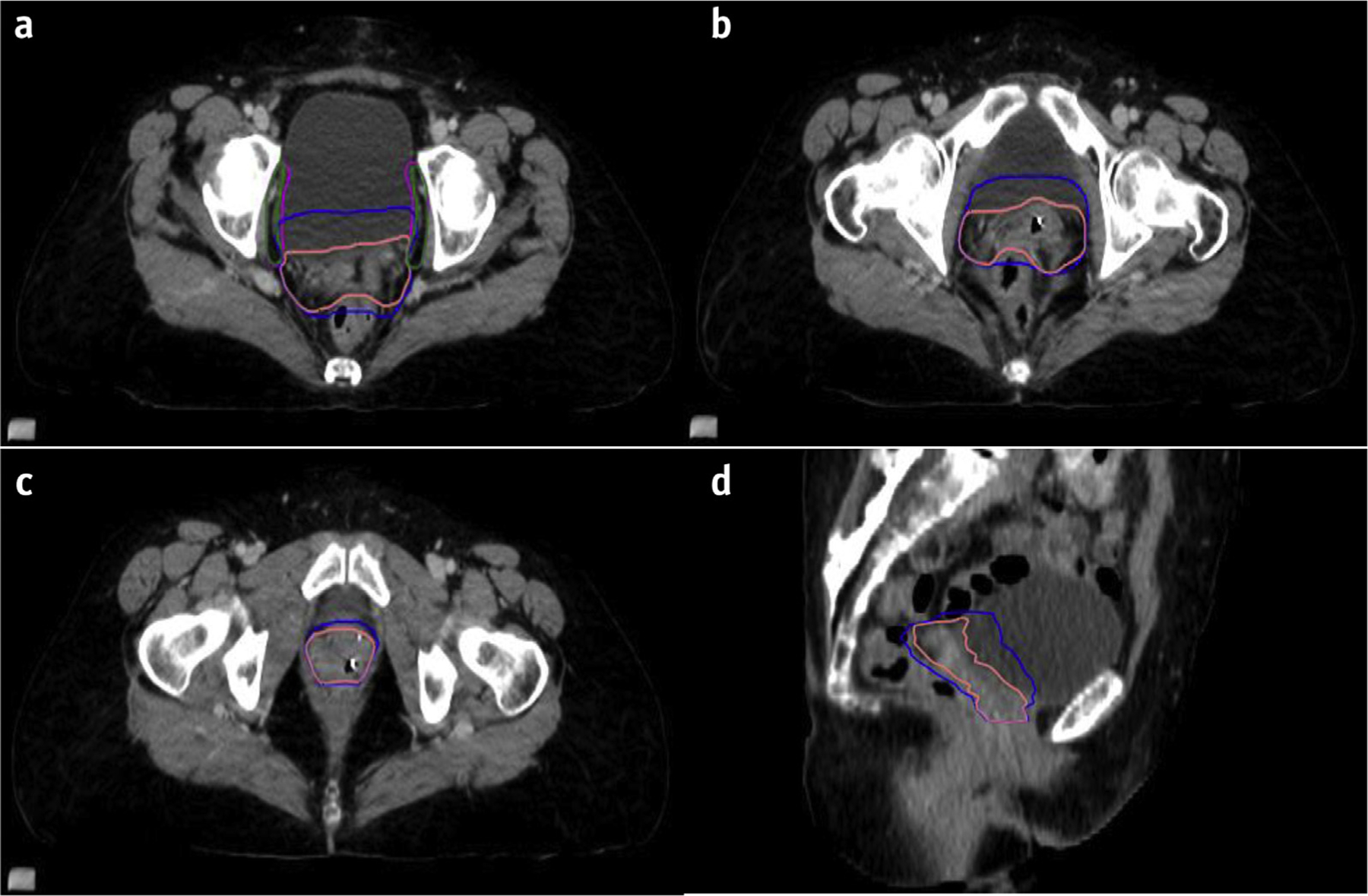
Use of internal target volume (ITVs). The vaginal ITV (blue) accounts for motion of the vaginal CTV (pink) in various states of bladder and rectal filling as show in in the upper, mid, and lower vagina (Fig. 5a-c) and on sagittal CT (Fig 5d). (a) The obturator nodal CTV (green) is carved out of bladder; however, an obturator nodal ITV (magenta) should also be considered, accounting for changes in bladder filling. (d) A sagittal view showing vaginal CTV and ITV. If ITVs are not used, then one should use a larger PTV to account for bladder and rectal filling.
Vaginal CTV
The vaginal CTV should include the proximal vagina and any paravaginal or retracted parametrial tissue that can be visualized on the planning CT (Fig. 6). The vaginal CTV is a static structure that does not take organ motion into account, whereas a vaginal ITV does. Delineation of a vaginal ITV was not included in the consensus contours for this atlas, but it is addressed in the Discussion and Fig. 5. The vaginal cuff can be visualized with the aid of surgical clips or a thin, flexible vaginal marker placed at time of CT simulation. The vaginal and parametria–paravaginal tissue often extends superior to the apex of the vaginal canal, particularly at the lateral aspects of the vaginal cuff owing to surgical techniques with approximation of the vaginal cuff (Fig. 6c). This has also been demonstrated on magnetic resonance imaging–based planning for vaginal brachytherapy.31 The vaginal CTV should extend posteriorly to the anterior rectal wall including approximately the anterior one third of the mesorectum. Additional mesorectum or residual uterosacral ligaments should be considered for patients with cervical cancer or endometrial cancer with parametrial or gross cervix involvement, but it can be omitted in the scenario of endometrial cancer with microscopic cervical stromal involvement. If there is no cervical involvement in postoperative endometrial cases, then one can consider less anterior mesorectum coverage. If the rectum is distended at time of simulation, one should consider inclusion of the anterior rectal wall or resimulate after bowel evacuation; this is addressed in the Discussion. The anterior border of the vaginal CTV is the posterior aspect of the bladder wall. The lateral extent is the medial border of the obturator nodal CTV or the obturator internus muscle. Inferiorly, the lateral aspect should conform to the medial border of the urogenital diaphragm (Fig 6). Approximately 3.5 to 4 cm of the proximal vaginal canal measured from the apex should be included in the vaginal CTV. For patients with extensive LVSI, positive vaginal margin, or adverse pathology, a longer length of the vagina may be treated. For patients with shorter vaginal canals, although often not visible, the urethra might approximate the inferior aspect of the CTV. For routine cases, the visible urethra would not be at risk and should be excluded from the CTV (Fig. 6d).
Fig. 6.
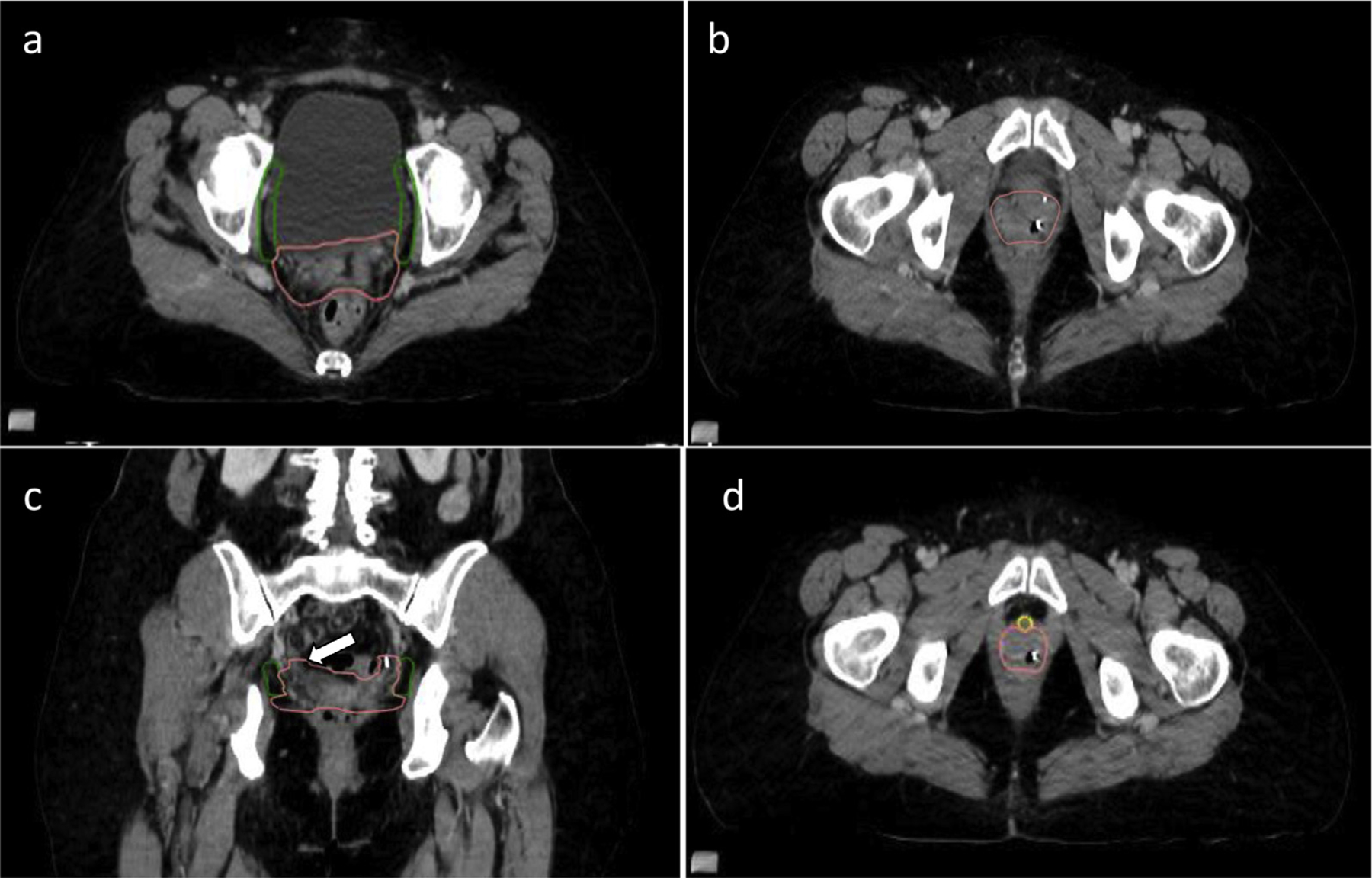
Vaginal clinical target volume (CTV). (a-d) The vaginal CTV (pink) includes the proximal vagina and any remaining parametrial tissue, and it should extend laterally to the obturator CTV (green) or (b) to the medial aspect of the obturator internus muscle. (c) On coronal view, one can appreciate the lateral “ears” of the vaginal cuff that should be included and can extend superior to the vaginal apex (white arrow). (d) For routine cases, the urethra (yellow) is not at risk and can be carved out of the inferior, anterior extent of the vaginal CTV.
Discussion
Over the last 2 decades, radiation therapy for gynecologic malignancies has evolved with the advent of IMRT and image-guided radiation therapy (IGRT). As such, it is important to accurately define and standardize radiation therapy target volumes. The use of pelvic IMRT has been shown in multiple retrospective and prospective studies to improve the therapeutic ratio of radiation therapy (RT) in gynecologic cancers by improving treatment related toxicity without compromising disease control.13,32–37 Reductions in the volumes of small bowel and rectum irradiated can reduce the incidences of acute and late gastrointestinal side effects.37,38 IMRT can also reduce the dose of radiation to bone marrow, reducing hematologic toxicity and, potentially facilitating safe delivery of concurrent chemoradiotherapy.39 Multiple phase 1 and 2 trials have demonstrated the feasibility of pelvic IMRT for gynecologic cancers using the previously published consensus guidelines.14,16,40,41 Recent results from the phase 3 NRG/RTOG 1203 (TIME-C) trial comparing 3D-CRT versus IMRT pelvic radiation for postoperative treatment of endometrial and cervical cancer demonstrated reduced acute and late toxicities and improved quality of life in those patients receiving IMRT compared with 3D-CRT.15,16
The use of expert-lead consensus guidelines in the delineation of target structures for IMRT in the postoperative gynecologic setting aids in the development of a more homogenous standardized treatment for this group of patients across institutions. This allows applicability of retrospective data and forms the basis of treatment defined in future multi-institutional prospective studies. These guidelines were created for a population of patients treated with IMRT or 3D conformal RT in the postoperative setting for endometrial and cervical cancer. However, for treatment planning of individual patients, these guidelines should be applied in conjunction with the treating physician’s judgment, taking into consideration individual patient anatomy and risk factors derived from the clinical and pathologic findings. The embryologic site of origin of gynecologic malignancies also plays an important role in the pattern of regional spread.42
As IMRT has become increasingly used for gynecologic malignancies, initial concerns that highly conformal treatment could compromise tumor control rates have been alleviated by the retrospective and prospective evidence described earlier. However, these trials were conducted in controlled settings with expert review of designated target volumes. Trial results cannot be generalized to everyday practice unless similar care is taken to define the at-risk volumes accurately in every case. There remains important inherent aspects of the treatment that one must take into account with IMRT planning. Because IMRT requires delineation of target volumes and avoidance structures, there has been some concern regarding increased rates of recurrence outside of the previously treated volumes with 2-dimensional or 3D techniques. Previous studies have demonstrated that a 7- to 10-mm margin around the iliac vessels encompassed >95% of the common iliac, internal iliac, medial, and anterior external iliac, and obturator lymph nodes, requiring a slightly larger margin around the presacral and anterolateral external iliac vessels. With small adjustments to this 7-mm vascular margin, nodal coverage increases to 99%.28 These margins were advocated in the prior contouring atlas and the current atlas, as early reported outcomes using the prior atlas demonstrate at least equivalent if not better locoregional disease control.13,36,40,43,44 As the adoption of IMRT in the postoperative setting for gynecologic malignancies became more commonplace, multiple retrospective and prospective studies reported on patterns of disease recurrence in the IMRT era. These studies demonstrated similar or lower rates of locoregional recurrences using IMRT compared with 3D-CRT, supporting the safety and efficacy of IMRT.41,43,44
Although these consensus guidelines provide direction for CTV contouring in a postoperative setting, there are several key points to consider. A successful CT simulation is required to appropriately contour target volumes. Table 2 provides a general set of recommendations for CT simulation of a postoperative gynecologic patient. An important consideration of contouring is the use of a vaginal ITV, which accounts for internal organ motion. Attention to organ motion is imperative with the use of IMRT compared with 3D-CRT in the pelvis. Anatomic displacement of the vaginal CTV due to changes in bladder and rectum volume can significantly alter the dose to targets and organs at risk. One can address this issue with the use of a vaginal ITV (Fig. 5) or a generous vaginal PTV if using strictly CTVs as described here.
Table 2.
Computed tomography simulation steps
| 1. Immobilize the patient in the supine position with a comfortably full bladder that she will be able to reproduce for each treatment. |
| • Possible bladder filling regimen: Instruct patient to void and then drink 500–750 mL of liquid 30–60 minutes before imaging. |
| 2. Consider placement of a thin vaginal canal marker to help delineate the vaginal canal and cuff, particularly if there are no surgical clips at the vaginal cuff. |
| • A thin, nonrigid marker can be used (a few millimeters in maximum diameter). |
| • Care should be taken to avoid displacement or deformation of the vaginal tissue when placing the marker. |
| 3. Consider addition of intravenous and oral contrast. This may help with delineation of targets and normal organs at risk. |
| 4. Consider simulating patient with both a full and empty bladder scan, particularly if planning to have a vaginal internal target volume. |
| • If doing a dual scan, consider intravenous contrast in either the full or empty bladder scan. |
| 5. When contouring, it is important to use the non–contrast intravenous scan to draw clinical treatment volumes, as intravenous contrast can cause vessel distention and lead to an unnecessarily large clinical treatment volume. |
When creating a vaginal ITV, scan the patient with the bladder both full and empty to capture the extreme of bladder volume changes and associated movement of the vaginal cuff. The ITV accounts for the motion of the vagina and parametria during these varying states of bladder filling. Variations in rectal volume should also be considered, although they are often more difficult to control. If a patient has a distended rectum at the time of simulation, the vaginal ITV should include the anterior one third to half of the rectum to account for the variation of an empty rectum during treatment. Figure 5 demonstrates comparisons of a vaginal CTV and the corresponding vaginal ITV that was created in this patient using both the bladder full and bladder empty scan (not shown) and accounting for rectal distension. A nodal ITV can be considered accounting for bladder filling and emptying. This is most pertinent in the obturator region, but overdistension of the bladder can lead to slight movement of other nodal regions. Figure 5a shows an obturator nodal CTV and obturator nodal ITV accounting for bladder motion.
Regarding PTV expansions, the size of the expansion depends on multiple factors, including institutional standards based on setup reproducibility, the type of image guidance used during treatment, and whether an ITV was considered. Generally, the recommendation is to have a uniform expansion of 5 to 10 mm around the nodal CTVs to create the nodal PTVs. A PTV expansion of 7 mm was used in the TIME-C randomized trial of IMRT in postoperative gynecologic cancers.15 A tighter vaginal PTV margin of 6 to 8 mm can be safe if using an ITV with daily cone beam CT image guidance ensuring accurate setup and bladder fill and rectal distension. A margin of 1.5 to 2.0 cm or greater would be recommended if not using an ITV and no or minimal image guidance. Dosimetric data and plan evaluations demonstrated movement of vaginal cuff fiducial markers up to approximately 1.5 cm throughout a course of postoperative IMRT, even with a strict bladder filling protocol, owing to changes in bladder and rectum volumes.45,46 This concept has also been studied in prostate cancer, in which bladder and rectal volumes can vary as much as 30% during treatment, leading to a redistribution of the target dose.47,48 These studies reinforce the importance of using a vaginal ITV, or a generous PTV if using a vaginal CTV, and image guidance with CBCT during treatment.
Future directions include evaluations of patterns of recurrence in the IMRT era of pelvic radiation, improving image guidance during radiation with possible adaptive planning, assessing the role of adjuvant pelvic IMRT in the sentinel node era of gynecologic surgery, and evaluating dose escalation potential as we continue to minimize toxicities associated with pelvic radiation with the use of IMRT.
Conclusion
Over the last decade, IMRT is increasingly used in the treatment of gynecologic cancers in the postoperative setting because of the improvements in treatment related toxicities while achieving comparable disease control. This report serves as an expert consensus for the delineation of the postoperative volumes used to form the updated NRG/RTOG atlas for postoperative gynecologic treatment. Further research into disease patterns of recurrence and additional avenues to reduce toxicities are eagerly awaited.
Acknowledgments
This project was supported by grants U10CA180868 (NRG Oncology Operations) and U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI).
Disclosures: N.A-R. reports grants paid to their institution from Stryker/Novadaq, Olympus, and Grail outside the submitted work and is funded in part by the National Institutes of Health/National Cancer Institute Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748). S.B. discloses the role of Assistant Medical Director for Via Oncology, and honoraria from Varian, Eisai, Xoft (DSMB), and the International Journal of Radiation Oncology. W.R.B. discloses an NCI IROC grant from the National Institutes of Health (NIH), during the time of the study. L.P. discloses advisory board and steering committee roles with BTG, Biocompatibles, an advisory board role with Sirtex, and moderator and speaker roles for ViewRay, outside the submitted work. W.S. discloses honoraria from Carl Zeiss, an advisory board role with Merck and Varian, and a cochair role with NRG Oncology, outside the submitted role.
References
- 1.ASTEC/EN.5 Study Group. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009;373:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987;60:2035–2041. [DOI] [PubMed] [Google Scholar]
- 3.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post operative radiation therapy in endometrial carcinoma. Lancet 2000;355:1404–1411. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–751. [DOI] [PubMed] [Google Scholar]
- 5.Greven K, Winter K, Underhill K, et al. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol 2006;103:155–159. [DOI] [PubMed] [Google Scholar]
- 6.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999; 340:1154–1161. [DOI] [PubMed] [Google Scholar]
- 7.Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–183. [DOI] [PubMed] [Google Scholar]
- 8.Chino JP, Jones E, Berchuck A, et al. The influence of radiation modality and lymph node dissection on survival in early-stage endometrial cancer. Int J Radiat Oncol Biol Phys 2012;82:1872–1879. [DOI] [PubMed] [Google Scholar]
- 9.Lee CM, Szabo A, Shrieve DC, et al. Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma. JAMA 2006;295:389–397. [DOI] [PubMed] [Google Scholar]
- 10.Harkenrider MM, Adams W, Block AM, et al. Improved overall survival with adjuvant radiotherapy for high-intermediate and high risk Stage I endometrial cancer. Radiother Oncol 2017;122:452–457. [DOI] [PubMed] [Google Scholar]
- 11.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 2006;65:169–176. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan AN, Moughan J, Miller BE, et al. NRG Oncology/RTOG 0921: a phase 2 study of postoperative intensity-modulated radiotherapy with concurrent cisplatin and bevacizumab followed by carboplatin and paclitaxel for patients with endometrial cancer. Cancer 2015;121:2156–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;52:1330–1337. [DOI] [PubMed] [Google Scholar]
- 14.Barillot I, Tavernier E, Peignaux K, et al. Impact of post operative intensity modulated radiotherapy on acute gastro-intestinal toxicity for patients with endometrial cancer: Results of the phase II RTCMIENDOMETRE French multicentre trial. Radiother Oncol 2014;111:138–143. [DOI] [PubMed] [Google Scholar]
- 15.Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol 2018;36:2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung A NRG Oncology-RTOG 1203, IMRT Improves Late Toxicity Compared to Conventional RT: An Update on NRG Oncology-RTOG 1203. Chicago, IL: Presented at ASTRO; 2019. [Google Scholar]
- 17.Small W Jr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation. IEEE Trans Med Imaging 2004;23:903–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Panici PB, Scambia G, Baiocchi G, et al. Anatomical study of para-aortic and pelvic lymph nodes in gynecologic malignancies. Obstet Gynecol 1992;79:498–502. [PubMed] [Google Scholar]
- 21.Keenan LG, Rock K, Azmi A, et al. An atlas to aid delineation of para-aortic lymph node region in cervical cancer: Design and validation of contouring guidelines. Radiother Oncol 2018;127:417–422. [DOI] [PubMed] [Google Scholar]
- 22.Takiar V, Fontanilla HP, Eifel PJ, et al. Anatomic distribution of fluorodeoxyglucose-avid para-aortic lymph nodes in patients with cervical cancer. Int J Radiat Oncol Biol Phys 2013;85:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eifel PJ, Klopp AH. Letter to the Editor: In reply to Keenan et al. Anatomic principles as the basis of target volume definition. Radiother Oncol 2019;136:198–199. [DOI] [PubMed] [Google Scholar]
- 24.de Boer S, Powell ME, Mileshkin LR, Creutzberg CL. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GOG 0249: A phase III trial of pelvic radiation therapy versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy in patients with high risk, early stage endometrial carcinoma. RTOG Endorsed; Version Date: January 2011. Available at: https://www.rtog.org/LinkClick.aspx?fileticketZfFkWdAUubC8%3D&tabidZ331. Accessed October 6, 2020.
- 26.GOG-0258: A randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs. carboplatin and paclitaxel for optimally debulked, advanced endometrial carcinoma. (NCT#00942357). NCI Version Date: 04/04/2014, Available at: https://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.5505. Accessed October 6, 2020.
- 27.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol 2017;18:384–392. [DOI] [PubMed] [Google Scholar]
- 28.Taylor A, Rockall AG, Reznek RH, et al. Mapping pelvic lymph nodes: Guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:1604–1612. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Rustum NR, Barakat RR. Observations on the role of circumflex iliac node resection and the etiology of lower extremity lymphedema following pelvic lymphadenectomy for gynecologic malignancy. Gynecol Oncol 2007;106:4–5. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman MS, Parsons M, Gunasekaran S, et al. Distal external iliac lymph nodes in early cervical cancer. Obstet Gynecol 1999;94:391–394. [DOI] [PubMed] [Google Scholar]
- 31.Chapman CH, Prisciandaro JI, Maturen KE, et al. MRI-based evaluation of the vaginal cuff in brachytherapy planning: Are we missing the target? Int J Radiat Oncol Biol Phys 2016;95: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heron DE, Gerszten K, Selvaraj RN, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: A comparative dosimetric study of dose-volume histograms. Gynecol Oncol 2003;91:39–45. [DOI] [PubMed] [Google Scholar]
- 33.Portelance L, Chao KS, Grigsby PW, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys 2001;51:261–266. [DOI] [PubMed] [Google Scholar]
- 34.Roeske JC, Lujan A, Rotmensch J, et al. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2000;48:1613–1621. [DOI] [PubMed] [Google Scholar]
- 35.Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys 2003;56:1354–1360. [DOI] [PubMed] [Google Scholar]
- 36.Beriwal S, Jain SK, Heron, et al. Clinical outcome with adjuvant treatment of endometrial carcinoma using intensity-modulated radiation therapy. Gynecol Oncol 2006;102:195–199. [DOI] [PubMed] [Google Scholar]
- 37.Beriwal S, Gan GN, Heron DE, et al. Early clinical outcome with concurrent chemotherapy and extended-field, intensity-modulated radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2007; 68:166–171. [DOI] [PubMed] [Google Scholar]
- 38.Shih KK, Hajj C, Kollmeier M, et al. Impact of postoperative intensity-modulated radiation therapy (IMRT) on the rate of bowel obstruction in gynecologic malignancy. Gynecol Oncol 2016;143: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mell LK, Sirak I, Wei L, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an International Multicenter Phase II Clinical Trial (INTER-TECC-2). Int J Radiat Oncol Biol Phys 2017;93:536–545. [DOI] [PubMed] [Google Scholar]
- 40.Jhingran A, Winter K, Portelance L, et al. A phase II study of intensity modulated radiation therapy to the pelvis for postoperative patients with endometrial carcinoma: radiation therapy oncology group trial 0418. Int J Radiat Oncol Biol Phys 2012;84: e23–e28. [DOI] [PubMed] [Google Scholar]
- 41.Viswanathan AN, Moughan J, Miller BE, et al. NRG Oncology/RTOG 0921: a phase 2 study of postoperative intensity-modulated radiotherapy with concurrent cisplatin and bevacizumab followed by carboplatin and paclitaxel for patients with endometrial cancer. Cancer 2015;87:S4–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eifel PJ, Klopp AH. Gynecologic Radiation Oncology: A Practical Guide. Philadelphia, PA: Wolters Kluwer; 2017;2–4. [Google Scholar]
- 43.Hajj C, Kollmeier MA, Gardner G, et al. Patterns of relapse in patients with endometrial cancer treated with postoperative intensity modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys 2015;93:3S. [Google Scholar]
- 44.Hasselle MD, Rose BS, Kochanski JD, et al. Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2011;80:1436–1445. [DOI] [PubMed] [Google Scholar]
- 45.Jurgenliemk-Schulz IM, Toet-Bosma MZ, de Kort GAP, et al. Internal motion of the vagina after hysterectomy for gynaecological cancer. Radiother Oncol 2011;98:244–248. [DOI] [PubMed] [Google Scholar]
- 46.Jhingran A, Salehpour M, Sam M, et al. Vaginal motion and bladder and rectal volumes during pelvic intensity-modulated radiation therapy after hysterectomy. Int J Radiat Oncol Biol Phys 2012;82:256–262. [DOI] [PubMed] [Google Scholar]
- 47.Roeske JC, Forman JD, Mesina CF, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys 1995;33:1321–1329. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z, Yang Z, Wang J, et al. Dosimetric impact of different bladder and rectum filling during prostate cancer radiotherapy. Radiat Oncol 2016;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]


