Abstract
Aims
To examine how circulating GLP-1 concentrations during liraglutide treatment relate to its therapeutic actions on glucose and weight, and to study the effects of liraglutide on other proglucagon-derived peptides (PGDPs), including endogenous GLP-1, GLP-2, glucagon, oxyntomodulin, glicentin, and major proglucagon fragment, which also regulate metabolic and weight control.
Materials and methods
Adults who were overweight/obese (BMI 27–40 kg/m2) with prediabetes were randomized to liraglutide (1.8mg/day) vs placebo for 14 weeks. We used specific assays to measure exogenous (liraglutide, GLP-1 agonist (GLP-1A)) and endogenous (GLP-1E) GLP-1, alongside 5 other PGDP concentrations during a mixed-meal tolerance test (MMTT) completed at baseline and at week 14 (liraglutide, n=16; placebo, n=19). Glucose during MMTT, steady-state plasma glucose (SSPG) concentration for insulin resistance, and insulin secretion rate (ISR) were previously measured. MMTT area-under-the-curve (AUC) was calculated for ISR, glucose, and levels of PGDPs.
Results
Participants on liraglutide vs placebo had significantly (p≤0.004) decreased weight (mean −3.6%, 95% CI [−5.2, −2.1]), SSPG (−32% [−43, −22]) and glucose AUC (−7.0% [−11.5, −2.5]), and increased ISR AUC (30%, [16, 44]). Treatment with liraglutide significantly (p≤0.005) increased exogenous GLP-1A AUC (median 310 vs 262 pg/mL × 8h at baseline but decreased endogenous GLP-1E AUC (13.1 vs 24.2 pmol/L × 8h at baseline)), as well as the 5 other PGDPs. Only glucagon AUC decreased in the placebo group (471 vs 594 pg/mL × 8h at baseline). GLP-1A AUC at study end was significantly (p≤0.04) linearly associated with % decrease in weight (r=−0.54) and SSPG (r=−0.59) and increase in ISR AUC (r=0.51) in the liraglutide group.
Conclusions
Circulating GLP-1A concentrations, reflecting liraglutide levels, predict improvement in weight, insulin action, and secretion in a linear manner. Importantly, liraglutide also downregulates all other PGDPs, normalization of the levels of which may provide additional metabolic benefits in the future.
Introduction
Liraglutide is currently approved for the treatment of type 2 diabetes and obesity. It is a prototype glucagon-like peptide 1 receptor agonist (GLP-1ra) and has 97% homology to native GLP-11. Native GLP-1 derives from proglucagon, which is also a prohormone for other peptides including GLP-2, glucagon, oxyntomodulin, glicentin, and major proglucagon fragment (MPGF). Aside from GLP-1 and glucagon, the actions and roles of the other proglucagon-derived peptides (PGDPs) remain to be fully elucidated but oxyntomodulin and glicentin have recently been proposed to have important metabolic roles2–4. Importantly, the effect of liraglutide treatment on these peptides remains unknown. In addition, the relationship between concentrations of exogenously administered GLP-1 and its actions, including on weight loss, glycemic control, insulin action, and secretion, has not been evaluated.
We have previously shown that treatment with liraglutide compared with placebo for 14 weeks significantly decreases body weight and insulin resistance5, increases insulin secretion rate (ISR), and lowers glucose concentrations during a mixed meal-tolerance test (MMTT) in overweight/obese individuals with prediabetes. This study aimed to quantitate the effect of treatment with liraglutide compared with placebo on GLP-1 levels using two assays—one that cross-reacts with liraglutide and one that does not—as well as to explore the effects of liraglutide treatment on other PGDPs which remains unknown. We secondarily aimed at directly evaluating the relationship between levels of GLP-1 derived using the two GLP-1 assays on the main physiological effects achieved with liraglutide treatment, including weight loss, glucose homeostasis, and changes in insulin action and secretion.
Materials and methods
Patient recruitment
The Stanford Institutional Review Board approved the study protocol, and all study participants provided written informed consent. The study was conducted according to the Declaration of Helsinki. To be eligible for the study, participants were required to be 40–70 years old, overweight or obese (body mass index (BMI) 27–40 kg/m2) and have prediabetes based on a 75-gram oral glucose tolerance test (OGTT, fasting glucose 5.6–6.9 mmol/L; 2-h glucose 7.8–11.0 mmol/L). Participants were otherwise healthy with no known cardiac, liver, or kidney disease. Participants were recruited from December 2009 to December 2012 at a single center (NCT01784965). All testing was completed in the Stanford Clinical and Translational Research Unit (CTRU) after fasting for 12 hours overnight.
Study Design
Participants were randomized to receive either liraglutide (n=35) or matching placebo (n=33) for 14 weeks. Liraglutide or matching placebo were injected subcutaneously daily before breakfast. Dose was escalated weekly from 0.6mg to 1.2mg to 1.8mg daily. Both participants and study staff (physicians, nurses, dietitian, and coordinators) were blinded to treatment assignment.
Weight-loss Intervention
All participants were required to attend weekly meetings with a dietitian for the first 4 weeks and then bimonthly. Participants were advised to decrease total energy intake by 500 kcal per day and to maintain baseline physical activity.
Insulin Suppression Test
Peripheral insulin resistance was directly measured using the modified version6 of the Insulin Suppression Test at baseline and after 14 weeks of liraglutide or placebo injections daily. After an overnight fast, participants had a 180-minute infusion of octreotide (0.27 mg/m2/min), insulin (32 mU/m2/min), and glucose (267 mg/m2/min). Blood was collected at 10-minute intervals from 150 to 180 minutes to determine the steady-state plasma glucose (SSPG) and insulin concentrations. Because steady-state insulin concentrations are similar among individuals, the SSPG concentration provides a direct measure of the ability of insulin to mediate disposal of an infused glucose load. Higher SSPG values, therefore, indicated a greater degree of peripheral insulin resistance.
Graded-glucose Infusion Test
ISRs were estimated from c-peptide concentrations measured during graded infusion of intravenous glucose. Briefly, the glucose infusion rate was started at 1mg/kg/min and increased every 40 minutes to 8mg/kg/min. Blood was drawn for C-peptide concentrations at baseline and twice before each rate change at times 30, 40, 70, 80, 110, 120, 150, 160, 190, 200, 230, and 240 minutes7.
Mixed-Meal Tolerance Test (MMTT)
The MMTT involved eating breakfast at 08:00 (20% of daily energy intake) and lunch at 12:00 (40% of daily energy intake). Each meal was prepared in the CTRU and composed of 43% carbohydrates, 42% fat, and 15% protein. Blood was drawn hourly starting from before breakfast at 08:00 to 16:00. Participants had MMTT at baseline and after 14 weeks of either liraglutide or placebo injections daily. Injection of liraglutide or placebo was given prior to breakfast for the end-of-study MMTT.
Assays
Samples from the MMTT were stored at −80°C after pretreatment with aprotinin and dipeptidyl peptidase-4 inhibitor. Complete samples were available for 35 participants (16 in the liraglutide and 19 in the placebo groups).
GLP-1 was quantified using two specific assays to measure exogenous (i.e., liraglutide) and endogenous GLP-1 concentrations, respectively. GLP-1A from Ansh Labs detects both endogenous GLP-1 and liraglutide in a dose-dependent manner (AL-172, dynamic range, 15–213 pg/mL. See Supplementary Appendix for further details). We also measured total GLP-1, referred to as GLP-1E, from separate samples using an ELISA Kit from Epitope Diagnostics (San Diego, CA, KT-876, Dynamic Range 0–54 pmol/L). GLP-1E measures endogenous GLP-1 (7–36) and (9–36) and does not cross-react with liraglutide.
Blood was also analyzed using commercially available immunoassays from Ansh Labs (Webster, TX) to measure c-peptide (AL-151, dynamic range 0.25–14.5 ng/mL) and PGDPs including GLP-2 (AL-174, 0.21–10 ng/mL), oxyntomodulin (AL-139, 4.2–316 pg/mL), glicentin (AL-185, 33.3–1666 pg/mL), glucagon (AL-157, 6.8–310 pg/mL), and MPGF (AL-175, 0.056–3.25 ng/mL). All samples were run neat in the ELISAs, and any samples reading over the curve in the assays were diluted 1:2 in calibrator A/sample diluent and re-assayed (See Supplementary Appendix for further details).
Glucose concentrations during the MMTT and Insulin Suppression Test were quantified by the oxidase method (Analyzer 2; Beckman, Brea, CA, USA). C-peptide concentrations during the graded-glucose infusion test and insulin concentration during the MMTT were measured at Washington University (St Louis, MO, USA) using radioimmunoassay (Millipore, St Charles, MO, USA). The intra and inter-assay coefficient of variation ranged between 4.7% and 9.7% for insulin and 5.2% and 10.9% for C-peptide, respectively.
Statistical Analysis
The aim of the current study was to compare the effects of liraglutide versus placebo on all PGDPs and to assess potential direct linear associations between GLP-1 levels achieved by exogenously administering GLP-1 and important metabolic outcomes. We calculated area-under-curve (AUC) using the trapezoidal method for all measures during the MMTT and for ISR during the graded-glucose infusion test. We used Shapiro-Wilk tests of normality and log-transformed any variables which were not normally distributed (Glucagon, MPGF, GLP-1E, GLP-2). Comparisons within and between groups were conducted using paired and independent t-tests, respectively. We also used mixed-model analyses and adjusted for baseline levels and weight loss to evaluate the effect of liraglutide on PGDFs independent of weight loss.
Given our previous findings that liraglutide treatment decreases weight and insulin resistance8, increases ISR, and lowers glucose concentration7, we also evaluated the association between GLP-1 AUC, using both assays, and percentage change in weight, SSPG, ISR, and glucose concentration during the MMTT. Thus, we evaluated Spearman’s correlation (r-value) between baseline and 14-week GLP-1 AUC and percentage change in weight, SSPG, ISR AUC, and glucose AUC.
All statistical analyses were conducted using SPSS (version 26 for Windows, SPSS, Chicago, IL, USA). P<0.05 was considered statistically significant and adjustments were not made for multiple testing given the independence of the hypotheses tested herein.
Results
We initially randomized 68 individuals to either liraglutide (n=35) or placebo (n=33). There were 24 participants on liraglutide and 27 on placebo who completed the study5. More participants on liraglutide (8 out of 11) vs placebo (none) discontinued intervention due to adverse events (mainly gastrointestinal)5. The current study includes a subset of individuals who had remaining samples from the MMTT (16 liraglutide, 19 placebo). These subjects were similar in age and BMI with subjects in the original cohort.
Baseline characteristics were comparable between the participants treated with liraglutide and placebo (Supplemental Table 1). In addition, similar to the original study5, individuals treated with liraglutide lost twice as much weight as those assigned to placebo injections (mean ± SD, −6.1 ± 1.9 vs −3.2 ± 2.2 kg, p<0.001), representing a greater mean decrease in baseline weight of 3.6%, (95% CI, −5.2 to −2.1) relative to placebo. In addition, individuals on liraglutide compared with placebo injections significantly decreased SSPG concentration (−3.8 ± 2.2 mmol/L vs −0.2 ± 1.8, p<0.001; relative mean difference of −32% [−43 to −22]) and increased insulin secretion rate AUC (481 ± 411 vs −122 ± 545 pmol/min × 4 hours, p<0.001; relative mean increase of 30% [16 to 44]), as previously reported5,7.
Figure 1 illustrates changes in GLP-1 concentration using two specific assays during the MMTT. As seen in Figure 1B, treatment with liraglutide increased GLP-1A levels, which detects liraglutide concentrations. In contrast, liraglutide treatment significantly decreased GLP-1E concentrations, which only measures endogenous GLP-1 concentrations.
Figure 1: Exogenous and endogenous GLP-1 levels at baseline and 14 weeks of liraglutide vs placebo administration.
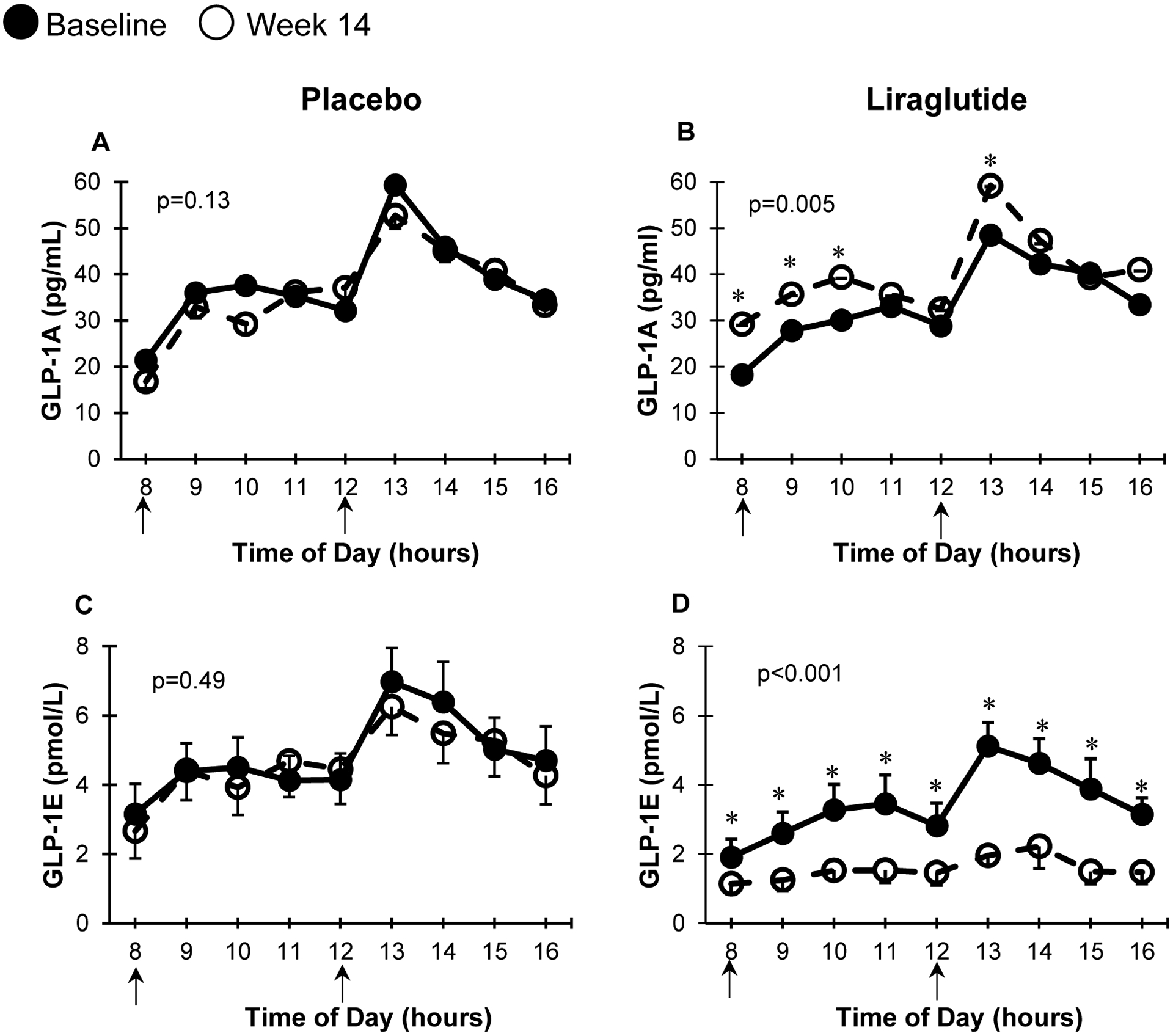
MMTT curves at baseline (solid circles) and 14 weeks (open circles) for GLP-1A and GLP-1E in participants receiving placebo (n=19, A and C) or liraglutide (n=16, B and D respectively). * represents p<0.05 at each particular time point using paired t-test. p for AUCs = statistical difference betweenbaseline and 14-week AUCs using paired t test. Arrows represent meals administered during the MMTT. Data presented in mean ± SEM (error bars).
Figure 2 and Figure 3 show changes in glucose and other PGDPs concentrations during the MMTT. Also summarized in Table 1, results reveal that treatment with liraglutide was associated with a significant (p ≤ 0.001) decrease in glucose AUC and all other PGDPs. In the placebo group, only glucagon significantly decreased after 14-weeks, and the mean decrease was not significantly different from those on liraglutide. As shown in Table 1, AUC changes associated with liraglutide treatment were significantly different from placebo, except for insulin, c-peptide, and glucagon.
Figure 2: MMTT concentrations of glucose, GLP-2, and oxyntomodulin.
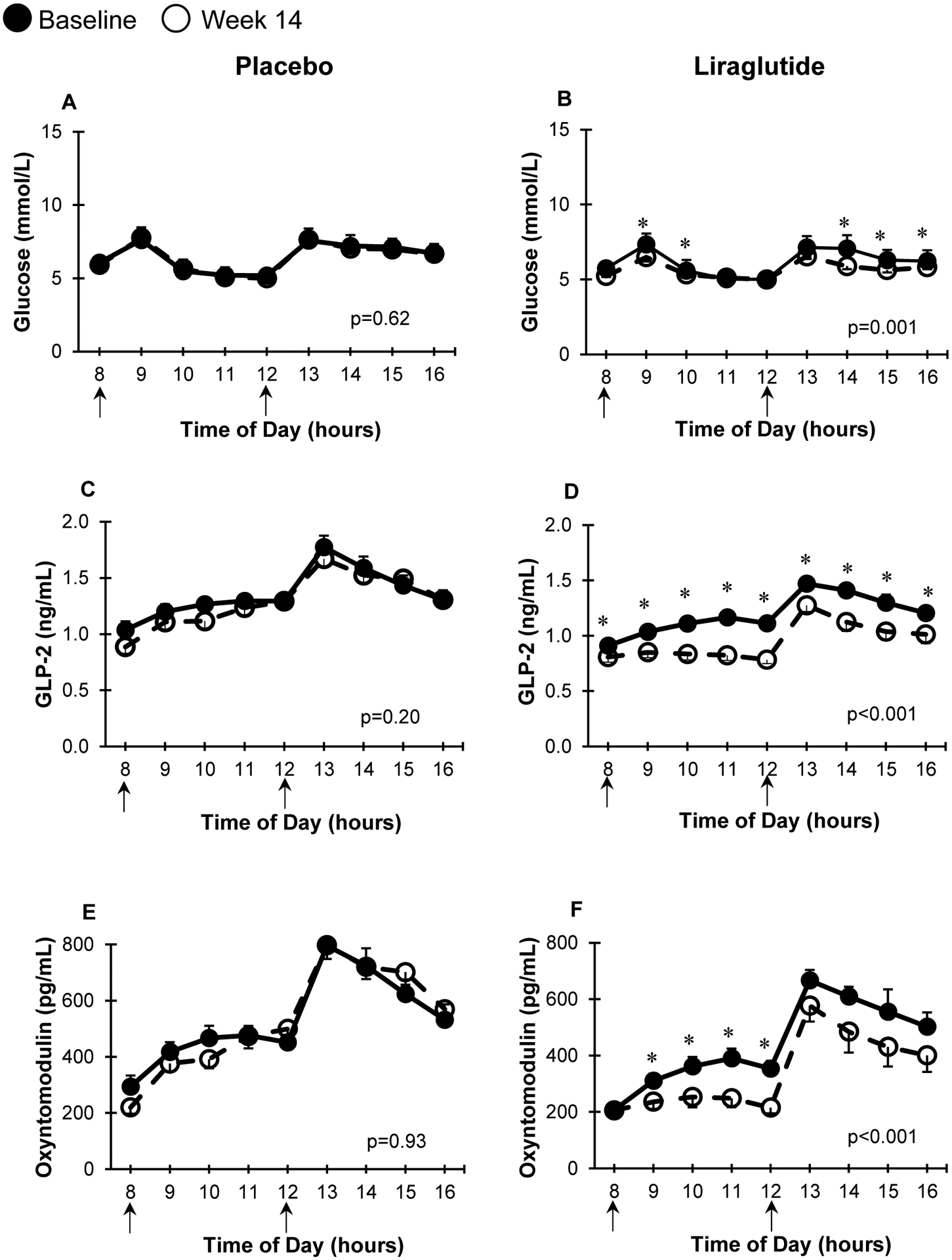
MMTT curves at baseline (solid circles) and 14 weeks (open circles) for glucose, GLP-2, and oxyntomodulin in participants receiving placebo (n=19, A, C, E) or liraglutide (n=16, B, D, F respectively). * represents p<0.05 at each particular time point using paired t-test. p for AUCs = statistical difference between baseline and 14-week AUCs using paired t test. Arrows represent meals administered during the MMTT. Data presented in mean ± SEM (error bars).
Figure 3: MMTT concentrations of glicentin, glucagon, and MPGF.

MMTT curves at baseline (solid circles) and 14 weeks (open circles) for glicentin, glucagon, and MPGF in participants receiving placebo (n=19, A, C, E) or liraglutide (n=16, B, D, F respectively). * represents p<0.05 at each particular time point using paired t-test. p for AUCs = statistical difference between baseline and 14-week AUCs using paired t test. Arrows represent meals administered during the MMTT. Data presented in mean ± SEM (error bars).
Table 1:
Proglucagon-derived peptides area-under-the-curve (AUC) at baseline and after 14 weeks of placebo or liraglutide treatment during the mixed-meal tolerance test
| Placebo (n = 19) | Liraglutide (n = 16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 14 weeks | Mean change relative to baseline† | p value | Baseline | 14 weeks | Mean change relative to baseline† | p value | p value for liraglutide vs placebo | p value Mixed Model‡ | |
| Glucose AUC mmol/L × 8h | 51.7 (49.2, 53.1) | 51.4 (49.1, 52.9) | −0.58 (−3.5, 2.3) | 0.62 | 50.0 (47.1, 52.3) | 45.4 (43.5, 48.8) | −7.6 (−11.4, −3.7) | 0.001 | 0.004 | 0.01 |
| Insulin AUC pmol/L × 8h | 3021 (2048, 5291) | 3146 (2334, 4473) | 2.3 (−11.5, 16.2) | 0.42 | 2556 (1504, 3752) | 2483 (1484, 3382) | −3.8 (−24.6, 16.9) | 0.18 | 0.59 | 0.98 |
| C-peptide AUC ng/mL × 8h | 71.6 (60.1–81.9) | 71.5 (59.1–80.3) | −0.02 (−7.8, 7.8) | 0.51 | 61.2 (52.9–75.9) | 58.2 (50.6–74.9) | −1.5 (−11.9, 8.9) | 0.41 | 0.81 | 0.26 |
| GLP-1A AUC pg/mL × 8h | 322 (260–365) | 294 (261–365) | −2.5 (−12.4, 7.4) | 0.13 | 262 (242–316) | 310 (266–376) | 18.0 (6.1, 29.9) | 0.005 | 0.002 | 0.01 |
| GLP-1E AUC pmol/L × 8h | 33.3 (23.9–44.2) | 33.6 (22.3–37.2) | −1.3 (−12.0, 9.5) | 0.49 | 24.2 (18.8–30.7) | 13.1 (5.7–18.8) | −58.2 (−70.2, −46.1) | <0.001 | <0.001 | 0.02 |
| GLP-2 AUC ng/mL × 8h | 10.7 (9.5–12.3) | 9.9 (8.5–12.3) | −3.2 (−10.2, 3.7) | 0.20 | 9.9 (8.8–10.5) | 7.9 (6.9–8.5) | −20.4 (−25.4, −15.4) | <0.001 | <0.001 | <0.001 |
| Oxyntomodulin AUC pg/mL × 8h | 4,166 (3,515–5,409) | 4,247 (3,730–5,191) | 2.1 (−6.3, 10.5) | 0.93 | 3,489 (2,886–4,349) | 2,702 (1,945–3,656) | −24.4 (−31.9, 17.0) | <0.001 | <0.001 | <0.001 |
| Glicentin AUC pg/mL × 8h | 2,468 (1,900–3,052) | 2,302 (2,209–3,054) | 4.0 (−6.6, 14.6) | 0.94 | 2,093 (1,696–2,514) | 1,618 (1,060–2,514) | −25.4 (−34.2, 16.6) | <0.001 | <0.001 | 0.001 |
| Glucagon AUC pg/mL × 8h | 594 (469–838) | 471 (378–716) | −12.5 (−22.2, −2.8) | 0.002 | 553 (410–671) | 396 (333–458) | −21.7 (−29.6, −13.9) | <0.001 | 0.13 | 0.69 |
| MPGF AUC ng/mL × 8h | 8.8 (6.9–10.7) | 7.7 (6.2–10.2) | −6.7 (−17.0, 3.7) | 0.07 | 9.2 (7.5–12.7) | 7.2 (5.9–9.9) | −22.1 (−27.5, −16.7) | <0.001 | 0.01 | 0.83 |
Peptide AUCs are median (interquartile range).
Mean change relative to baseline, % (95% CI)
p value for liraglutide vs placebo after adjustment for baseline AUC and weight loss using Mixed Model analyses
We also conducted mixed-model analyses to compare PGDPs at 14-weeks adjusting for baseline PGDPs and percentage weight loss. There was a significant (p<0.05) effect of group, time, and group*time interaction for both GLP-1 measures, GLP-2, oxyntomodulin, and glicentin, suggesting a direct effect of liraglutide treatment to change these PGDPs independent of weight loss. MPGF was not significantly different between groups at 14-weeks when adjusted for weight loss indicating a potential weight loss mediated change. Glucagon levels remained similar between groups at 14-weeks.
Finally, we evaluated the association between baseline and end-of-study GLP-1 AUC (using both assays) and percentage change in weight, SSPG, ISR AUC, and glucose AUC during the MMTT. As seen in Figure 4, there was a significant, linear and inverse association between GLP-1A AUC at 14-weeks and percentage change in weight (B) and SSPG (D) in the liraglutide group but not in the placebo group. In addition, GLP-1A AUC at 14-weeks was associated with a linear percentage increase in ISR AUC (F) in the liraglutide group. There was no significant association between GLP-1A AUC at 14-weeks and percentage change in glucose AUC in either group (data not shown).
Figure 4: Relationship between GLP-1A AUC and percentage change in weight, insulin resistance (SSPG), and ISR after liraglutide vs placebo administration.

Data are shown for % change in weight, SSPG, and ISR at 14 weeks relative to baseline in participants receiving placebo (n=19, A, C, E) or liraglutide (n=16, B, D, F respectively). r for Spearman correlation coefficient, p represents significance for correlation between GLP-1A and percentage change.
Baseline GLP-1A was inversely associated with percentage change in glucose AUC in the placebo group (r= −0.55, p=0.01) but not in the liraglutide group (r=−0.22, p=0.44). There was no significant association between GLP-1E AUC at baseline or GLP-1E at end-of-study and weight, SSPG, ISR AUC, or glucose AUC (data not shown).
Discussion
We demonstrate herein clinically novel and mechanistically useful physiological effects of liraglutide treatment. Firstly, injection of liraglutide daily for 14 weeks, concomitant with pharmacological increase of exogenous GLP-1, is associated with a decrease in all PGDPs including endogenous GLP-1. Decreases in PGDPs, except for glucagon and MPGF, are independent of weight loss, indicating a probable direct effect of GLP-1 receptor agonists to decrease endogenous GLP-1, GLP-2, oxyntomodulin, and glicentin i.e. the PGDPs processed in the intestines versus glucagon and MPGF that are processed in pancreatic alpha cells9. Secondly, GLP-1A AUC at the end of the study, an indicator of liraglutide concentration, is linearly associated with many physiologically important GLP-1 actions. These results provide biologic evidence and confirmation for the clinically observed dose-response effect of liraglutide8 and other GLP-1 receptor agonists10 to promote weight loss and increase insulin secretion rate11.
By using two different GLP-1 assays, we fully demonstrate the pharmacological effects of a GLP-1 receptor agonist. We show that liraglutide treatment significantly suppresses endogenous GLP-1 concentrations as illustrated by lowered GLP-1E levels throughout the MMTT (figure 1D). Thus, similar to other PGDPs, endogenous GLP-1 levels decrease following liraglutide treatment. While exogenous GLP-1 therapy may be expected to negatively feedback on PGDPs, the cleavage/production of which occurs in the intestines, these effects have never been previously shown. The differential regulation of PGDPs processed in the intestines versus those processed in the pancreas which are influenced only by weight loss has also not been shown earlier and is thus novel information on human physiology and potentially therapeutics. Kramer et al. reported an increase in endogenous GLP-1 response during chronic liraglutide treatment in patients with type 2 diabetes, when GLP-1 concentrations were measured during a 75-gram OGTT and after 24 hours from last liraglutide injection12. OGTTs were conducted at baseline, 12, 24, 36, 48 weeks while on liraglutide and after 2 weeks off liraglutide. Although the increase in GLP-1 was shown up to 48 weeks on liraglutide treatment, GLP-1 concentrations unfortunately were not reported after stopping liraglutide to determine persistence or not. The investigators did report that improvements in insulin secretory function on liraglutide treatment were already lost at 2 weeks after stopping liraglutide13.
In contrast to endogenous GLP-1E concentrations, GLP-1A concentrations appropriately increase with liraglutide treatment (figure 1B), and GLP-1A AUC at the end of the study associates with known actions of GLP-1 receptor agonists. Specifically, GLP-1A AUC at the end of the study was associated with weight loss and improvement in insulin action and insulin secretion. Although there is a specific assay for liraglutide14 and liraglutide concentration increases with drug dose15, no prior study has reported a direct and linear association of liraglutide concentrations with its metabolic actions. Nevertheless, our results are consistent with clinical trials of liraglutide showing greater weight loss with higher liraglutide dose8. Our data also are consistent with infusion studies of GLP-1 showing dose-response of GLP-1 concentration and glucose-stimulated insulin secretion rate11. We did not show any significant associations between GLP-1A AUC at the end of the study and any changes in glucose AUC during the MMTT, which may be attributed to conducting the study in a population with prediabetes with relatively good glucose control.
In the past, assays for PGDPs have been challenging16. Even for established peptides such as glucagon, assays have affected the interpretation of the effects of liraglutide treatment. Kramer et al. initially published that chronic liraglutide treatment was associated with an increase in glucagon concentration after a 75-gram oral glucose challenge in patients with type 2 diabetes17. However, reanalysis using a different assay showed no increase in glucagon levels18. Kramer et al hypothesized that cross-reactivity of the glucagon assays with other PGDPs especially oxyntomodulin may have contributed to the difference in results18. In the current study, we used a state of the art novel well-validated glucagon assay which did not cross-react with oxyntomodulin, and showed a non-differential decrease in glucagon concentrations during the MMTT after weight loss in both the placebo and actively treated groups, which is consistent with a previous weight-loss trial19 and a recent meta-analysis showing a decrease in fasting glucagon after weight loss20. We also demonstrate that MPGF is regulated in a manner similar to glucagon in humans.
Unlike the other PGDPs which are predominately secreted from L-cells of the distal intestine, glucagon, as well as MPGF, is secreted from pancreatic alpha cells9. The prevailing view is that GLP-1 suppresses glucagon via indirect mechanisms9. GLP-1 stimulates delta cells to secrete somatostatin and beta cells to secrete insulin as well as zinc and amylin; all of these products have been shown to inhibit glucagon secretion9. Although there is controversy regarding the prevalence and relevance of GLP-1 receptors on alpha-cells, a recent study in mice with alpha-cell specific GLP-1 receptor knockout suggested direct effects of GLP-1 to inhibit glucagon secretion21. In addition, the study highlighted the bidirectional effects of GLP-1 depending on glucose concentration with GLP-1 stimulating glucagon secretion at lower glucose concentration and inhibiting glucagon at higher glucose concentration. Our study highlights that the changes in glucagon and MPGF may predominately occur through weight loss. Weight loss has pleiotropic effects and may alteralpha-cell secretory products via improvements in insulin sensitivity and reduction in insulin concentration19,20 or additional hormones. Decrease in glucagon concentration also has been suggested to be a defense mechanism to increase hunger during energy restriction20. Further investigations are needed.
Liraglutide appears to regulate L-cell derived PGDFs more directly than alpha-cell derived PGDFs. While mechanisms are not completely understood, GLP-1 has been shown to negatively feedback on its own secretion as well as on other L-cell products, including GLP-222–24. This negative L-cell feeback is likely mediated by increase in intestinal somatostatin22–24 but delay in gastric emptying may also play a role25.
Although GLP-1 receptor agonists are currently approved for the treatment of type 2 diabetes and obesity, other PGDPs are also being actively pursued such as glucagon and oxyntomodulin for obesity and related metabolic disorders. In two recent studies, increases in oxyntomodulin, as well as glicentin, were found to independently predict weight loss after bariatric surgery and were better predictors of weight loss than GLP-12,4. Glucagon concentrations after bariatric surgery have been variable2,26 and likely do not associate with surgery-associated weight loss. In our study, treatment with a GLP-1 receptor agonist downregulates all other PGDPs. Whether pharmacological increase of the other peptides will further improve glucose regulation and/or attenuate the weight plateau associated with GLP-1 receptor agonist therapy, as indicated by our data herein, will need further investigation. A recent study did report promising effects of dual GLP-1 and glucagon receptor agonist on reducing glucose and body weight27, but more studies are needed to understand any effects of combination treatments with glicentin and/or oxyntomodulin on human metabolism and to better understand benefits and risks.
Our study had a relatively small sample size and included only those individuals who tolerated liragutide; however, the sample size was sufficient to illustrate the significant effects of liraglutide treatment on multiple hormones derived from proglucagon, which has never been studied previously. We used two different assays to differentiate between endogenous and exogenous GLP-1. Importantly we showed a significant direct and linear relationship between exogenously administered GLP-1A AUC and several of its important metabolic actions.
In conclusion, our study reveals two new interesting effects of liraglutide treatment. Liraglutide concentration predicts GLP-1 related actions in a linear manner, while simultaneously downregulating all other PGDPs processed by the intestines but not the ones processed in the pancreas, which are regulated by weight loss. These observations raise the notion that pharmacological increase of the circulating levels of the other PGDPs, in addition to exogenous administration of GLP-1 analogs, may mitigate the weight loss plateau, which predictably occurs over time. Whether there would be differential weight loss based on addition of intestinal versus pancreatic PGDPs also deserve further investigation. Thus, future studies need to evaluate how multiple agonists with the properties of PGDP molecules can improve, alone or in combination, body weight, and glycemic/ metabolic control
Supplementary Material
Acknowledgements
The authors thank the study participants.
Funding
The original study was funded by the American Diabetes Association (7-09-NOVO-15). SHK is partially funded by the Stanford Diabetes Research Center (P30DK116074) and Bose Family Foundation. CSM is partially funded by NIH K24DK081913.
Footnotes
Ethics approval and consent
The study protocol (#17394) was approved by the Stanford Institutional Review Board and all study participants provided written informed consent. The study was conducted according to the Declaration of Helsinki.
Data accessibility: The data that support the findings of this study are available from the authors on reasonable request.
Publication Ethics: We confirm that this manuscript has not been published elsewhere and is not being considered elsewhere.
Trial registration: NCT01784965 (Retrospectively registered in ClinicalTrials.gov on February 6, 2013). The full trial protocol may be accessed on: https://clinicaltrials.gov/ct2/show/NCT01784965.
Conflict of interest statement
AK, BK, GS work for Ansh Labs. CSM has been a shareholder of and reports grants through his institution and personal consulting fees from Coherus Inc and personal consulting fees from Pangea Inc, reports grants through his institution and personal consulting fees from Eisai and Novo Nordisk, reports personal consulting fees and in kind support with research reagents from Ansh Labs, reports personal consulting fees from Genfit, P.E.S., Loumos, Intercept, Astra Zeneca, Aegerion and Regeneron, reports in kind support (educational activity meals at and through his institution) from Amarin, Janssen, Boehringer Ingelheim and travel support and fees from TMIOA, the California Walnut Commission and the Cardiometabolic Health Conference.
References
- 1.Jacobsen LV, Flint A, Olsen AK, Ingwersen SH. Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin Pharmacokinet. 2016;55(6):657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perakakis N, Kokkinos A, Peradze N, et al. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: Evidence from two independent trials. Metabolism. 2019;101:153997. [DOI] [PubMed] [Google Scholar]
- 3.Perakakis N, Mantzoros CS. The role of glicentin and oxyntomodulin in human metabolism: new evidence and new directions. J Clin Endocrinol Metab. 2020; 105(8):e3003–e3005. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen MS, Ritz C, Wewer Albrechtsen NJ, Holst JJ, le Roux CW, Sjödin A. Oxyntomodulin and Glicentin May Predict the Effect of Bariatric Surgery on Food Preferences and Weight Loss. J Clin Endocrinol Metab. 2020;105(4). [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Abbasi F, Lamendola C, et al. Benefits of Liraglutide Treatment in Overweight and Obese Older Individuals With Prediabetes. Diabetes Care. 2013;36(10):3276–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30(5):387–392. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Liu A, Ariel D, et al. Pancreatic beta cell function following liraglutide-augmented weight loss in individuals with prediabetes: analysis of a randomised, placebo-controlled study. Diabetologia. 2013;57(3):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616. [DOI] [PubMed] [Google Scholar]
- 9.Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frias JP, Wynne AG, Matyjaszek-Matuszek B, et al. Efficacy and safety of an expanded dulaglutide dose range: A phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab. 2019;21(9):2048–2057. [DOI] [PubMed] [Google Scholar]
- 11.Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380–386. [DOI] [PubMed] [Google Scholar]
- 12.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. Chronic liraglutide therapy induces an enhanced endogenous glucagon-like peptide-1 secretory response in early type 2 diabetes. Diabetes Obes Metab. 2017;19(5):744–748. [DOI] [PubMed] [Google Scholar]
- 13.Retnakaran R, Kramer CK, Choi H, Swaminathan B, Zinman B. Liraglutide and the preservation of pancreatic β-cell function in early type 2 diabetes: the LIBRA trial. Diabetes Care. 2014;37(12):3270–3278. [DOI] [PubMed] [Google Scholar]
- 14.Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45(2):195–202. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard RV, Petri KC, Jacobsen LV, Jensen CB. Liraglutide 3.0 mg for Weight Management: A Population Pharmacokinetic Analysis. Clin Pharmacokinet. 2016;55(11):1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wewer Albrechtsen NJ, Veedfald S, Plamboeck A, et al. Inability of Some Commercial Assays to Measure Suppression of Glucagon Secretion. J Diabetes Res. 2016;2016:8352957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. The Impact of Chronic Liraglutide Therapy on Glucagon Secretion in Type 2 Diabetes: Insight From the LIBRA Trial. J Clin Endocrinol Metab. 2015;100(10):3702–3709. [DOI] [PubMed] [Google Scholar]
- 18.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. Impact of the Glucagon Assay When Assessing the Effect of Chronic Liraglutide Therapy on Glucagon Secretion. J Clin Endocrinol Metab. 2017;102(8):2729–2733. [DOI] [PubMed] [Google Scholar]
- 19.Villareal DT, Banks MR, Patterson BW, Polonsky KS, Klein S. Weight loss therapy improves pancreatic endocrine function in obese older adults. Obesity (Silver Spring). 2008;16(6):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvestre MP, Goode JP, Vlaskovsky P, McMahon C, Tay A, Poppitt SD. The role of glucagon in weight loss-mediated metabolic improvement: a systematic review and meta-analysis. Obes Rev. 2018;19(2):233–253. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Parajuli KR, Fava GE, et al. GLP-1 Receptor in Pancreatic α-Cells Regulates Glucagon Secretion in a Glucose-Dependent Bidirectional Manner. Diabetes. 2019;68(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen L, Hartmann B, Bisgaard T, Mineo H, Jørgensen PN, Holst JJ. Somatostatin restrains the secretion of glucagon-like peptide-1 and −2 from isolated perfused porcine ileum. Am J Physiol Endocrinol Metab. 2000;278(6):E1010–1018. [DOI] [PubMed] [Google Scholar]
- 23.Brubaker PL, Efendic S, Greenberg GR. Truncated and full-length glucagon-like peptide-1 (GLP-1) differentially stimulate intestinal somatostatin release. Endocrine. 1997;6(1):91–95. [DOI] [PubMed] [Google Scholar]
- 24.Chisholm C, Greenberg GR. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am J Physiol Endocrinol Metab. 2002;283(2):E311–317. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Pilichiewicz AN, Feinle-Bisset C, et al. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med. 2012;29(5):604–608. [DOI] [PubMed] [Google Scholar]
- 26.Jorsal T, Wewer Albrechtsen NJ, Christensen MM, et al. Investigating Intestinal Glucagon After Roux-en-Y Gastric Bypass Surgery. J Clin Endocrinol Metab. 2019;104(12):6403–6416. [DOI] [PubMed] [Google Scholar]
- 27.Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607–2618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


