Figure 5. PAF-related toxicities are generalizable to IVJP and MC3 LNP nanocarriers and are enhanced in systemically-inflamed 4T1 tumor-bearing mice.
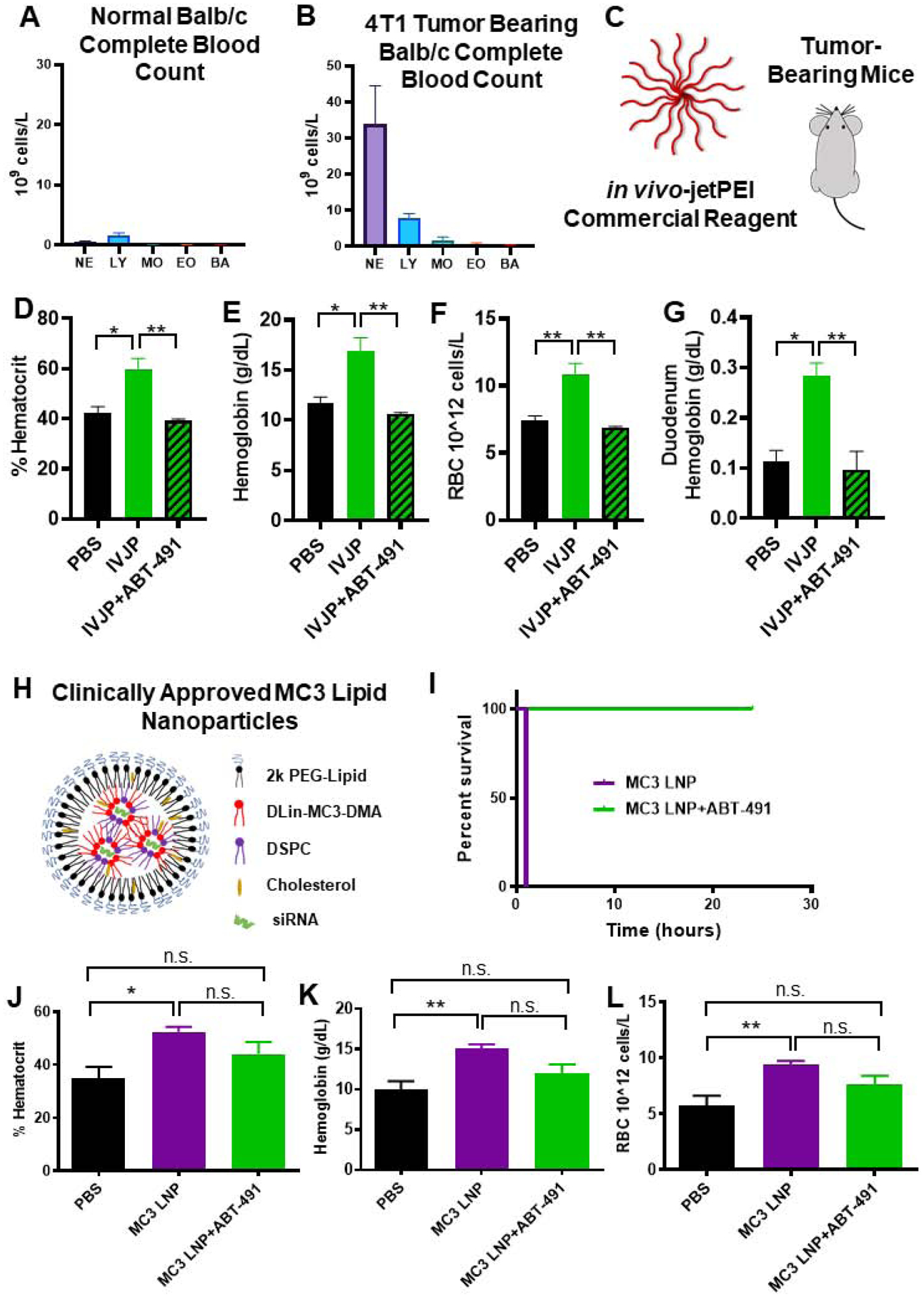
A-B) Comparison of leukocytes in complete blood counts of untreated normal BALB/c mice or 4T1 tumor-bearing mice. C) Tumor-bearing mice were injected with 2 mg/kg IVJP D-G) Blood hematocrit, hemoglobin, red blood cell concentration, and duodenum hemoglobin concentration for tumor-bearing mice treated with saline, IVJP, or ABT-491 pre-treatment in combination with IVJP (n=3 mice per group). H) Schematic illustrating components of Patisiran LNP formulation. I) Survival curve for mice injected intravenously with 5 mg/kg MC3 LNP formulation with either saline or ABT-491 pre-treatment. (n=2 mice per group) J-L) Hematocrit, hemoglobin, and red blood cell concentration values of mice treated with saline, MC3 LNPs, or MC3 LNPs with ABT-491 pre-treatment (n=4–5). Blood measurements were made 20 min after administration of MC3 LNPs. (* p<0.05, **p<0.01) NE, neutrophils; LY, lymphocytes; MO, monocytes; EO, eosinophils; BA, basophils.
