Abstract
Summary
As part of the innate immune response, the host withholds metal micronutrients such as Cu from invading pathogens, and microbes respond through metal starvation stress responses. With the opportunistic fungal pathogen Candida albicans, the Cu sensing transcription factor Mac1p governs the cellular response to Cu starvation by controlling Cu import. Mac1p additionally controls reactive oxygen species (ROS) homeostasis by repressing a Cu-containing superoxide dismutase (SOD1) and inducing Mn-containing SOD3 as a non-Cu alternative. We show here that C. albicans Mac1p is essential for virulence in a mouse model for disseminated candidiasis and that the cellular functions of Mac1p extend beyond Cu uptake and ROS homeostasis. Specifically, mac1Δ/Δ mutants are profoundly deficient in mitochondrial respiration and Fe accumulation, both Cu-dependent processes. Surprisingly, these deficiencies are not simply the product of impaired Cu uptake; rather mac1Δ/Δ mutants appear defective in Cu allocation. The respiratory defect of mac1Δ/Δ mutants was greatly improved by a sod1Δ/Δ mutation, demonstrating a role for SOD1 repression by Mac1p in preserving respiration. Mac1p down-regulates the major Cu consumer SOD1 to spare Cu for respiration that is essential for virulence of this fungal pathogen. The implications for such Cu homeostasis control in other pathogenic fungi are discussed.
Abbreviated Summary
Across numerous fungi, the Cu-sensing transcription factor Mac1p induces transcription of the Cu import machinery during Cu limitation. In the opportunistic fungal pathogen Candida albicans, Mac1p has the added function of regulating homeostasis of reactive oxygen species involving down-regulation of the major Cu consumer Sod1p. This regulation of Sod1p has the added purpose of sparing Cu for cytochrome C oxidase essential for mitochondrial respiration and for virulence in a murine model of disseminated candidiasis.
Keywords: Candida albicans, copper, iron, cell respiration, superoxide dismutase
Introduction
Transition metals represent a double-edged-sword to biological systems; they are required for life but also can become toxic if not properly managed. Because of this dual nature, homeostasis of transition metals in biological systems is tightly controlled. Cu is one such metal that has this dual property of being both essential and potentially toxic. Cu is required as a redox cofactor for numerous enzymes involved in oxygen chemistry, but can also have deleterious side chemistry (Turski & Thiele, 2009, Festa & Thiele, 2011, Tan et al., 2014, Chillappagari et al., 2010, Liochev & Fridovich, 2002). Unicellular microbes are particularly vulnerable to fluxes in environmental metals and have evolved sophisticated means of regulating Cu homeostasis.
In the bakers’ yeast Saccharomyces cerevisiae Cu homeostasis is controlled by two Cu binding transcription factors: Ace1p and Mac1p (Keller et al., 2005). Ace1p senses high, toxic Cu concentrations and activates Cu detoxification machinery involving genes encoding Cu-binding metallothioneins (Culotta et al., 1994, Thiele, 1988). In times of Cu limitation, Mac1p activates transcription of genes for Cu uptake including the high affinity Cu permeases and cupric reductases (Gross et al., 2000). Variations on this theme of sensing and responding to extremes in Cu can be found throughout yeast species. The pulmonary fungal pathogen Cryptococcus neoformans uses the Cu-sensing transcription factor Cuf1p which bears homology to both S. cerevisiae Ace1p and Mac1p. Cuf1p activates both Cu uptake and Cu detoxification genes in response to Cu limitation and Cu excess, respectively (Garcia-Santamarina et al., 2018, Ding et al., 2011, Waterman et al., 2007, Waterman et al., 2012). On the other hand the pathogenic and saprotrophic fungi Aspergillus fumigatus has retained separate Ace1/Mac1 systems but in this fungus, AfMac1p has evolved to independently upregulate genes involved in Fe uptake as well as Cu uptake (Park et al., 2018, Kusuya et al., 2017, Cai et al., 2017, Park et al., 2017). The opportunistic human fungal pathogen Candida albicans also has separate Mac1p and Ace1p Cu regulators and the core functions of Mac1p have been retained, namely transcriptional activation of Cu uptake genes including the CTR1 Cu permease and FRE7 cupric reductase (Woodacre et al., 2008). However, C. albicans Mac1p has additionally evolved to regulate anti-oxidant systems including superoxide dismutase enzymes (SOD).
SOD metalloenzymes utilize redox active co-factors such as Mn and Cu to disproportionate superoxide anion free radicals and therefore play important roles in metabolism of reactive oxygen species (ROS) (Sheng et al., 2014). Eukaryotes typically contain a Cu/Zn-SOD1 in the cytosol and mitochondrial intermembrane space, and a distinct Mn-SOD2 in the mitochondrial matrix. Mitochondrial SOD1 and SOD2 ensure protection against respiratory chain superoxide, while cytosolic Cu/Zn-SOD1 can participate in cell signaling involving ROS (Weisiger & Fridovich, 1973, Broxton & Culotta, 2016, Sturtz et al., 2001, Jaarsma et al., 2001, Okado-Matsumoto & Fridovich, 2001, Reddi & Culotta, 2013, Montllor-Albalate et al., 2019, Juarez et al., 2008). Apparently unique to C. albicans and closely related fungi is a second Mn containing Sod3p in the cytosol (Lamarre et al., 2001). During Cu limitation, C. albicans Mac1p transcriptionally represses Cu-Sod1p and induces Mn-Sod3p to maintain cytosolic levels of SOD (Li et al., 2015, Broxton et al., 2018, Broxton & Culotta, 2016). Additionally, Mac1p induces a mitochondrial alternative oxidase (AOX2) to compensate for loss of mitochondrial Cu/Zn SOD1 (Woodacre et al., 2008, Broxton & Culotta, 2016). C. albicans Mac1p clearly participates in ROS homeostasis as well as Cu uptake.
The Mac1-Cu response is utilized by C. albicans during pathogenesis. In a murine model of disseminated candidiasis, where the kidney is the major target organ, kidney Cu levels fluctuate such that at early time points Cu levels are high, but decrease later in infection (Li et al., 2015, Mackie et al., 2016, Besold et al., 2018, Culbertson et al., 2020). C. albicans is able to sense these changes in Cu and exhibits abundant expression of SOD1 at early stages of infection, and then repression of SOD1 and transcriptional induction of SOD3 and CTR1 later in infection when Cu levels decrease (Li et al., 2015, Mackie et al., 2016, Besold et al., 2018). Clearly C. albicans senses a Cu starved microenvironment in murine kidneys and is responding using Mac1p. It was not known if this Mac1p response plays a role in virulence in this model. Furthermore, the full impact of Mac1p on cell physiology is not understood. Is the function of Mac1p limited to cell surface Cu uptake and ROS homeostasis? It was unclear what, if any, additional genes are Mac1p targets in C. albicans.
In these studies, we investigate the requirement for C. albicans Mac1p in pathogenesis and its expanded role in regulating metal homeostasis and anti-oxidant enzymes. We find that Mac1p does indeed contribute to pathogenesis in a murine model of disseminated candidiasis and that its cellular function extends beyond Cu uptake and reactive oxygen homeostasis. Cells lacking Mac1p are severely compromised in both mitochondrial respiration and Fe accumulation, both a product of disrupted Cu homeostasis. Loss of Cu uptake in mac1Δ/Δ mutants cannot totally explain these deficiencies; rather mac1Δ/Δ mutants are defective in intracellular distribution of Cu to essential targets including Cu-requiring cytochrome oxidase (COX) for respiration. We provide evidence that Mac1p control of anti-oxidant genes through down-regulation of SOD1 not only maintains oxidative stress protection, but also spares Cu for mitochondrial COX. Mac1p helps maintain respiration essential for pathogenesis in the face of limiting host copper.
Results and Discussion
The mac1 strain has a virulence defect in a murine model of disseminated candidiasis
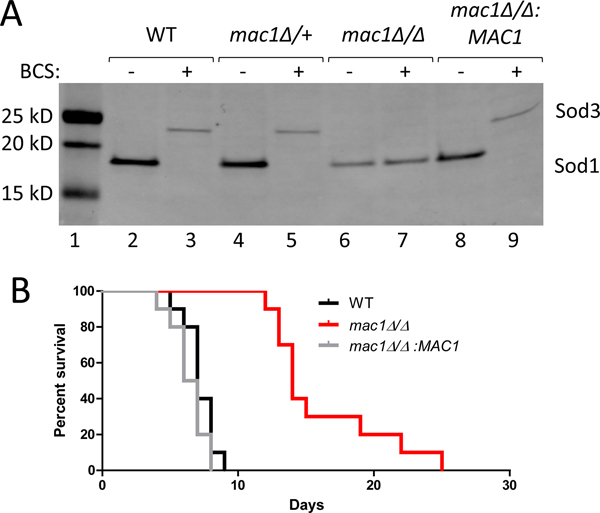
We sought to determine whether the Mac1p response to Cu during C. albicans invasion of the kidney was essential for pathogenesis. For these studies, we generated a mac1Δ/Δ mutation in a C. albicans clinical isolate lacking auxotrophic markers. Both mac1Δ/+ heterozygous and mac1Δ/Δ homozygous mutations were generated in the SC5314 background using the SAT1 flipper technique (Reuss et al., 2004). We validated that mac1Δ/Δ homozygotes, but not mac1Δ/+ heterozygotes, behaved similarly to a mac1Δ/Δ homozygous deletion in the SN152 background (Li et al., 2015), and are unable to switch from Cu containing Sod1p to Mn containing Sod3p during Cu starvation, as induced by the Cu(I) chelator bathocuproinedisulfonic acid (BCS) (Fig. 1A, lane 7). This defect was corrected by integrating a single copy of MAC1 into its native locus (Fig. 1A, lane 9). We used both the mac1Δ/Δ deletion strain and the reintegrated MAC1 rescue for virulence studies in a murine model of disseminated candidiasis where the kidneys are the major target organs and where tissue Cu levels decline during infection (MacCallum & Odds, 2005, Li et al., 2015, Mackie et al., 2016, Besold et al., 2018, Culbertson et al., 2020). As seen in Fig. 1B, female mice infected with WT C. albicans by lateral tail vein injection succumbed to the infection by 9 days. The mac1Δ/Δ strain displayed a stark virulence defect (Fig. 1B) with mac1Δ/Δ infected mice displaying a mean survival of 15 days post-infection. Virulence was fully restored by reintegration of MAC1 (Fig. 1B). Thus, Mac1p plays an important role in fungal virulence in disseminated candidiasis. Recent studies by Khemiri have shown role for C. albicans Mac1p in adherence to a human colon epithelial cell line in vitro, consistent with the importance of Mac1p in fungal growth and cell invasion (Khemiri et al., 2020).
Figure 1. Generation of a mac1Δ/Δ mutant and virulence in a murine model of disseminated candidiasis.
(A) Shown is immunoblot analysis of Sod1p and Sod3p for the indicated C. albicans strains using anti-SOD1 and anti-SOD3 antibodies as described in Experimental Procedures. (B) Survival curves are shown with groups of 10 female mice infected with 2×105 cells of the indicated strains as outlined in Experimental Procedures. There was a statically significant difference (p<0.0001) between mice infected with WT and those infected with mac1Δ/Δ but not between WT and mac1Δ/Δ:MAC1 (p=0.15). Statistical significance of survival curves was determined by the log-rank (Mantel-Cox) test. Strains utilized: WT, SC5314; mac1Δ/+, EC003; mac1Δ/Δ, EC004; mac1Δ/Δ:MAC1, EC005.
The effects of mac1Δ/Δ mutations on fungal copper accumulation and mitochondrial respiration
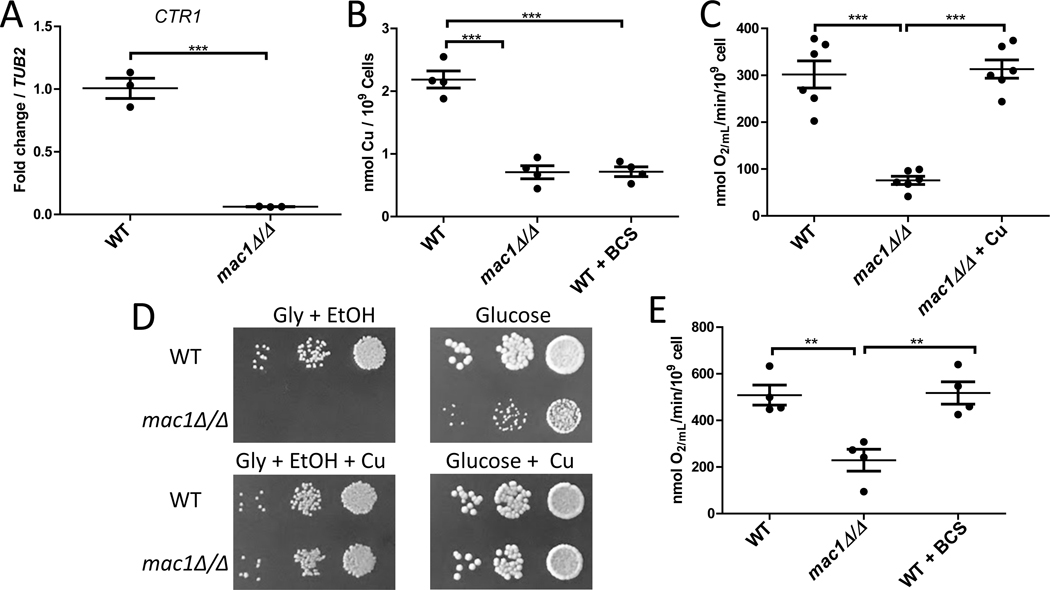
The basis for the virulence defect of mac1Δ/Δ strains was unclear. We therefore examined in detail the effects of MAC1 loss in cultures of C. albicans in vitro. Aside from regulating SOD1 and SOD3, Mac1p has been reported to induce the Cu permease gene CTR1 when cells are deprived of extracellular Cu (Woodacre et al., 2008). To directly monitor CTR1 expression we examined transcript levels by qRT-PCR. In enriched YPD media that was not depleted for Cu, CTR1 mRNA levels in the mac1Δ/Δ mutant were significantly lower than that of WT cells (Fig. 2A). Therefore Mac1p not only upregulates CTR1 with Cu starvation, but is also needed for basal levels of CTR1 expression when extracellular Cu is available. Consistent with this low basal level of CTR1 expression, mac1Δ/Δ mutants displayed ≈25–30% of the total intracellular Cu of WT cells (Fig. 2B, also see Figs. 3C and S1A). This low level of intracellular Cu in mac1Δ/Δ cells is similar to WT cells treated with 400 μM of the Cu chelator BCS (Fig. 2B).
Figure 2. The Cu deficiency and respiratory defects of mac1Δ/Δ strains.
(A) Expression of CTR1 mRNA was analyzed by qRT-PCR and results normalized to TUB2. Results are shown for three independent cultures. Expression of CTR1 in the mac1Δ/Δ strain was significantly different as determined by two-tailed student’s T test (p=0.0003). (B) Total cellular Cu levels were measured by AAS in the indicated strains. Shown are the results of 4 independent cultures across 2 experimental trials. (C,E) KCN-inhibitable oxygen consumption was measured by a Clark-type electrode in the indicated strains. “+ Cu” and “+BCS” indicates cells cultured and assayed for oxygen consumption in the presence of 50 μM CuSO4 and 400 μM BCS, respectively. Results are from 4–6 cultures across 2–3 experimental trials. (D) 5 μL of a cell solution containing 0.01, 0.001, and 0.0001 OD600/mL of the designated strains were plated on YPGE (“Gly + EtOH”) or YPD (“Glucose”) plates. Where indicated, media was supplemented with 100 μM CuSO4. Statistical significance for B,C,E was determined by one way ANOVA with Tukey posttest; **p≤0.01, ***p≤0.001. The absence of a bracket indicates that comparisons are not significantly different. Strains utilized are as described in Fig. 1. With all the data points, the wide bar reflects the mean value and the standard error of the mean (SEM) is shown with error bars.
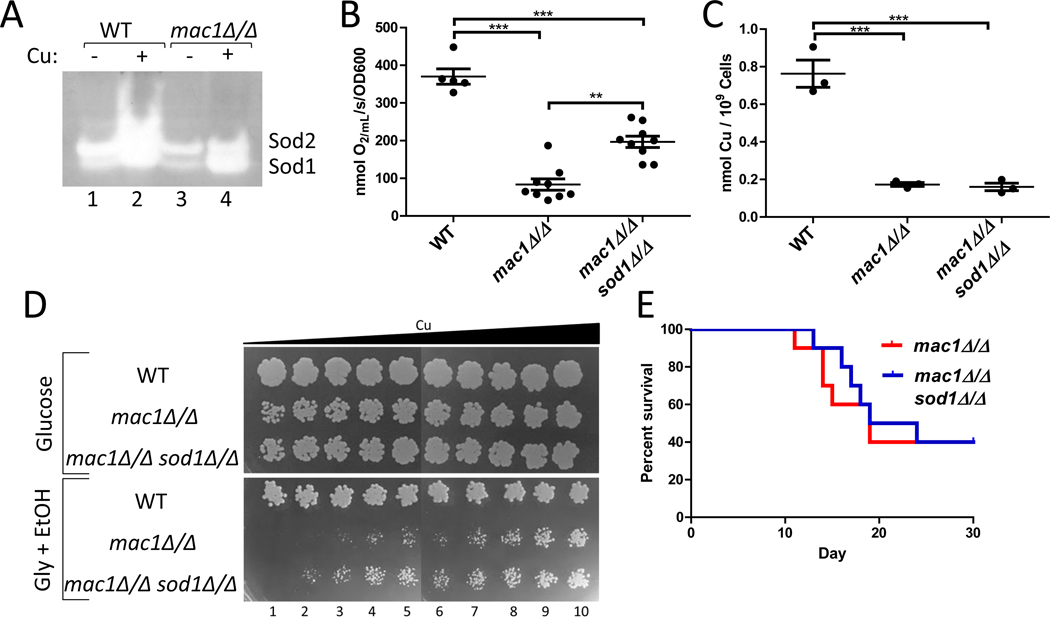
Figure 3. Deletion of SOD1 helps alleviate the respiratory deficiency of the mac1Δ/Δ strain.
(A) SOD activity was analyzed by the native gel assay as described in Experimental Procedures. Shown are results from cells grown in the presence or absence of 1 mM CuSO4. The mac1Δ/Δ strain exhibits Sod1 activity even without Cu supplements to the growth media. (B,C) Oxygen consumption (B) and total Cu levels (C) were measured in the indicated strains as described in Fig. 2C and 2B, respectively. Statistical significance was determined by one-way ANOVA with Tukey posttest; **p≤0.01, ***p≤0.001. The absence of a bracket indicates that comparisons are not significantly different. The individual data points represent 5–9 or 3 independent cultures in parts B or C respectively; the wide bar shows the mean and error bars represent SEM. (D) 10 μL of a cell solution containing 0.0005 OD600/mL of the indicated strains in PBS and increasing concentrations of Cu was spotted onto YPD (“Glucose”) or YPGE (“Gly + EtOH”). The cell solutions for columns 2–10 were supplemented with 50 μM, 75 μM, 100 μM, 125 μM, 150 μM, 175 μM, 200 μM, 250 μM¸ and 1000 μM CuSO4, respectively. (E) Shown are survival curves of groups of 10 female mice infected with 2×105 cells of the designated strains. There was no statically significant difference (p=0.51) between female mice infected with mac1Δ/Δ and those infected with mac1Δ/Δ sod1Δ/Δ strains. Statistical significance of survival curves was determined by the log-rank (Mantel-Cox) test. Strains utilized: WT, SC5314; mac1Δ/Δ, EC006; mac1Δ/Δ sod1Δ/Δ, EC007.
We tested whether this low level of intracellular Cu in mac1Δ/Δ mutants impacted mitochondrial respiration, a Cu-dependent process essential for virulence of C. albicans (Bambach et al., 2009, Becker et al., 2010, Aoki et al., 1990). Cytochrome c oxidase (COX) of the electron chain is a Cu-requiring complex (Turski & Thiele, 2009, Festa & Thiele, 2011) and can be monitored by measuring the rate of cyanide sensitive-oxygen consumption. As seen in Fig. 2C, COX-respiration in the mac1Δ/Δ strain was drastically reduced compared to the WT strain. This defect is indeed due to Cu deficiency, as oxygen consumption levels were restored to WT levels in cells supplemented with high Cu in the growth medium (Fig. 2C).
Since C. albicans is dependent on respiration for growth in vivo (Bambach et al., 2009, Becker et al., 2010, Aoki et al., 1990), we tested whether the lowered oxygen consumption in mac1Δ/Δ cells impacted respiration-dependent growth in vitro. C. albicans relies on respiration in the presence of non-fermentable carbon sources such as glycerol and ethanol. As seen in Fig. 2D top, mac1Δ/Δ strains failed to grow when glycerol and ethanol are provided as sole carbon sources, compared to growth on glucose that supports both fermentation and respiration. This requirement of Mac1p for growth on non-fermentable carbon sources is consistent with findings of mac1Δ/Δ mutants in an independent C. albicans strain background (BWP17) (Marvin et al., 2004). As with oxygen consumption, the respiratory defect of mac1Δ/Δ mutants was totally rescued by supplementation with Cu salts (Fig. 2D bottom). Thus, the respiratory deficiency of mac1Δ/Δ mutants as seen in Fig. 2C is indeed sufficient to block respiration-dependent growth as might occur in vivo during infection.
To test if low intracellular Cu by itself can explain the respiratory deficiency of mac1Δ/Δ cells, we quantified COX-respiration in WT cells that accumulate virtually identical low levels of total intracellular Cu through treatment with 400 μM BCS (Fig. 2B). Surprisingly, these Cu-depleted WT cells showed no defect in respiration (Fig. 2E). Unlike mac1Δ/Δ mutants, COX-respiration in WT cells appears resilient to Cu deprivation. Hence, the respiration defect of mac1Δ/Δ mutants cannot be explained by low levels of total intracellular Cu. Instead, the mutants may be unable to properly allocate Cu to COX in the mitochondria.
Contribution of SOD1 to the respiratory defect of mac1Δ/Δ cells
We addressed whether the apparent mac1Δ/Δ defect in Cu allocation to COX is due to improper regulation of SOD1. Sod1p is an abundant cellular protein that can account for 10–20% of the total intracellular Cu in yeast strains (Rae et al., 1999). Since mac1Δ/Δ mutants cannot repress SOD1 gene expression, Sod1p polypeptide levels remain unaffected in these Cu starved cells (Fig. 1A, lane 7, and (Li et al., 2015)). Most importantly, Sod1p retains a certain level of activity in mac1Δ/Δ cells (Fig. 3A, lane 3). In spite of very low intracellular Cu, Sod1p is still receiving Cu in mac1Δ/Δ cells.
To test whether the residual Cu bound to Sod1p in mac1Δ/Δ mutants is preventing Cu activation of COX, we generated a mac1Δ/Δ sod1Δ/Δ double mutant using a CRISPR based approach for C. albicans (Nguyen et al., 2017). When we assayed COX respiration, we found that deletion of SOD1 significantly ameliorated the oxygen consumption defect of mac1Δ/Δ mutants (Fig. 3B). This rescue of respiration by the sod1Δ mutation occurred without any increases in total intracellular Cu (Fig. 3C). Therefore, the presence of residual Sod1p in Cu-starved mac1Δ/Δ cells appears to function as a Cu sink, sequestering Cu away from COX. This notion of Sod1p limiting Cu accessibility to COX is consistent with our previous studies in WT cells where inappropriate over-expression of SOD1 lowered COX respiration (Broxton & Culotta, 2016). We conclude that Mac1p in C. albicans has evolved to maintain COX activity essential for growth and pathogenesis in at least two ways: by inducing Ctr1p-dependent Cu transport and by down-regulating Sod1p, a major Cu consumer. The regulation of anti-oxidant enzymes by C. albicans Mac1p not only serves to maintain oxidative stress protection and ROS signaling during Cu starvation (Broxton et al., 2018, Broxton & Culotta, 2016), but also facilitates Cu sparing for COX to help maintain mitochondrial respiration. A similar mechanism may extend to other organisms as well. In C. neoformans, the Cuf1p Cu sensor represses Cu/Zn SOD1 during Cu starvation (Garcia-Santamarina et al., 2018), which may also be part of a Cu sparing pathway for maintaining respiration or other essential Cu-dependent processes. The notion of Cu sparing for mitochondrial respiration has also be proposed for certain marine microorganisms. During Cu limitation, the photosynthetic algae Chlamydomonas reinhardtii degrades Cu containing plastocyanin for photosynthesis and Cu is prioritized for mitochondrial COX (Kropat et al., 2015, Merchant et al., 1991, Merchant & Bogorad, 1986). Down regulation of plastocyanin during Cu limitation has also been observed in diatoms (Hippmann et al., 2017).
Evidence for non-Sod1 factors in Mac1p-maintenance of respiration
It is important to note that loss of SOD1 only partly rescues COX-dependent respiration in mac1Δ/Δ mutants, but does not restore oxygen consumption to WT levels (Fig. 3B). Is this level of restored oxygen consumption sufficient to support respiration-dependent growth? As seen in Fig. 3D, deletion of SOD1 did not restore growth of mac1Δ/Δ strains on non-fermentable carbon sources without Cu supplements (Fig. 3D column 1); however the mac1Δ/Δ sod1Δ/Δ mutant did exhibit improved growth compared to the mac1Δ/Δ strain when low levels of Cu salts were added to the growth media (Fig. 3C, column 2–6). Thus, loss of SOD1 can improve respiration-dependent growth of a mac1Δ/Δ strain, but only under specific conditions of Cu availability. We also tested the effects of Sod1p loss in the disseminated model of candidiasis, where respiration is essential for virulence (Bambach et al., 2009, Becker et al., 2010, Aoki et al., 1990) and where the kidney is the major site of infection (MacCallum & Odds, 2005). Previous studies have shown that Cu availability becomes low in the kidney during C. albicans invasion (Li et al., 2015, Mackie et al., 2016, Culbertson et al., 2020, Besold et al., 2018), and with these Cu-limited conditions, loss of SOD1 may not be sufficient to support respiration-dependent growth. Indeed, as shown in Fig. 3E, there was no significant difference between mouse survival in mice infected with mac1Δ/Δ or mac1Δ/Δ sod1Δ/Δ strains. However, as a potential caveat to these studies, sod1Δ/Δ strains by themselves are defective in virulence in this mouse model (Hwang et al., 2002) and consistent with this, we observed a slight trend towards decreased virulence in the mac1Δ/Δ sod1Δ/Δ strain compared to the single mac1Δ/Δ mutant. In any case, our cell culture studies (Fig. 3C and 3D) demonstrate that Mac1p repression of SOD1 is just one method for preserving COX-respiration and other factors may contribute as well.
mRNA profile changes associated with mac1Δ/Δ mutations
To search for other factors that may contribute to the mac1Δ/Δ defect, we used an RNA-seq approach to identify possible targets of Mac1p regulation. For these studies, we compared mac1Δ/Δ to WT cells treated with 400 μM of the Cu(I) chelator BCS. As seen in Fig. S1A, these two experimental groups have similarly low levels of intracellular Cu (see also Fig. 2B). Any variations in gene expression patterns can therefore be ascribed specifically to the loss of Mac1p, not variations in Cu accumulation.
The possible targets of Mac1p-repression were defined as those genes repressed ≈ 3-fold or greater with BCS, but either unchanged or elevated in a mac1Δ/Δ strain. As shown in Fig. S1B, only three genes fit this category and of these, the most strongly repressed by Cu starvation is SOD1. Similar findings were obtained with C. neoformans, where the most prominent target of Cuf1-repression during Cu starvation is SOD1 (Garcia-Santamarina et al., 2018). In the case of C. albicans, other genes down-regulated by BCS but not in mac1Δ/Δ mutants include AAH1, which encodes an adenine deaminase (Uhl et al., 2003, Garcia-Sanchez et al., 2005), and FGR23, implicated in the regulation of filamentous growth (Uhl et al., 2003) (Fig. S1B). Neither AAH1 or FGR23 are predicted to encode cuproproteins, nor contain consensus sequences for Mac1p binding (Woodacre et al., 2008, Khemiri et al., 2020) downstream of the start site as is the case for SOD1 (Li et al., 2015), or in upstream promoter sequences. It is therefore possible that SOD1 is the only gene repressed by Mac1p in C. albicans, highlighting the importance of SOD1 repression during Cu starvation. As a possible caveat to our search of consensus binding sequences, C. albicans Mac1p may be promiscuous in its DNA recognition site, as has been observed for C. neoformans Cuf1p (Garcia-Santamarina et al., 2018) and A. fumigatus Mac1p (Kusuya et al., 2017, Park et al., 2018).
We also interrogated possible gene targets of Mac1p activation. Such genes should be upregulated upon treatment with BCS but not in mac1Δ/Δ strains. Indeed, we identified many known targets of Mac1p including genes for Cu uptake (CTR1, FRE7, FRE30), and genes that substitute for Sod1p loss (SOD3, AOX2) (Fig. S1C) (Woodacre et al., 2008, Li et al., 2015, Khemiri et al., 2020). Additionally genes encoding hexose sugar transporters and hyphal specific genes (e.g., ECE1, HWP1, ALS3) were among those uniquely induced by BCS (Fig. S2A and Table S1); none of which contained obvious Mac1p binding consensus sequences. We have previously shown that sod1Δ/Δ deletions induce hexose transporters, reflecting a role of Sod1p in glucose signaling (Broxton et al., 2018); the absence of Sod1p in BCS-treated WT cells (Fig. 1A) likely accounts for induction of sugar transporters in these cells. The induction of hyphal specific genes is consistent with some development of filamentation observed in Cu-starved WT cells, but not in the mac1Δ/Δ strain (Fig. S2B). Cu starvation is known to induce filamentation in C. albicans in a Mac1p-dependent manner (Marvin et al., 2004, Huang et al., 2006).
Fe-starvation state of mac1Δ/Δ mutants
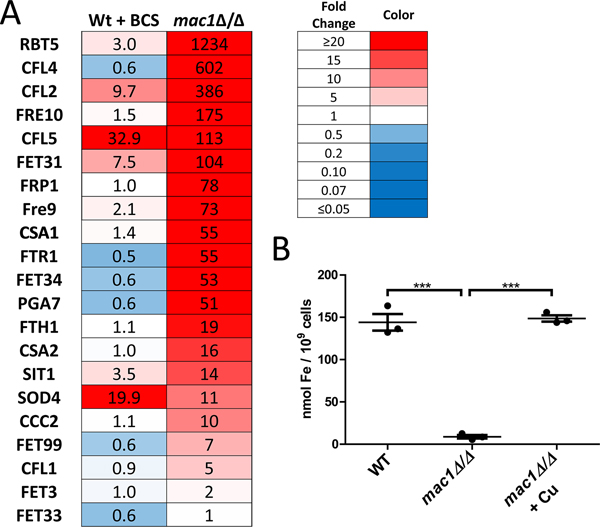
RNA-seq analysis indicated that mac1Δ/Δ mutants are not only starved for Cu but also Fe. Specifically, we observed a large number of genes induced in mac1Δ/Δ cells consistent with a transcriptional Fe-starvation stress response. Gene Ontology (GO) analysis revealed that many genes induced in the mac1Δ/Δ strain were related to ribonucleoprotein complex biogenesis, which is regulated by the HAP43/CCAAT binding protein Fe regulatory complex of C. albicans (Fig. S2A) (Singh et al., 2011). We observed strong induction of genes involved in reductive Fe uptake, heme acquisition and the siderophore transporter SIT1 (Fig. 4A), all of which are known be induced under Fe starvation stress conditions (Chen et al., 2011). While this manuscript was in its final stages of preparation, Khemiri et. al. published a study showing a similar high induction of specific Fe transport genes in mac1Δ/Δ cells from the background strain SN125 (Khemiri et al., 2020).
Figure 4. mac1Δ/Δ mutants are severely starved for Fe.
(A) The fold change in mRNA levels as determined by RNA-seq is shown for genes involved in Fe transport in C. albicans WT + BCS and in mac1Δ/Δ cells, both compared to untreated WT cells. (B) Intracellular levels of Fe were measured by a colorimetric BPS-based assay as described in Experimental Procedures. Where designated, cultures were supplemented with 100 μM CuSO4 (“+ Cu”). Values are from 3 independent cultures. Statistical significance was determined by one-way ANOVA with Tukey posttest; ***p≤0.001. Strains utilized: WT, SC5314; mac1Δ/Δ, EC004.
Regulation of Fe transport genes has also been reported with A. fumigatus Mac1p (Afmac1), however the role of Afmac1 in Fe homeostasis appears opposite to that of C. albicans Mac1p. AfMac1 is an activator of Fe uptake genes and loss of Afmac1p results in a decreased expression of Fe transporters (Park et al., 2018), unlike the pronounced induction of the Fe transport regulon with C. albicans mac1Δ/Δ mutations (Fig. 4A). C. albicans Mac1p is either a repressor of Fe uptake or the effects of mac1Δ/Δ are an indirect consequence of Cu starvation. We favor the latter. Cu is important for Fe acquisition and in yeast species including C. albicans, FET multicopper oxidases drive Fe uptake into the cell (Ziegler et al., 2011, Mamouei et al., 2017). Consistent with this requirement for Cu in Fe uptake we find that C. albicans mac1Δ/Δ mutants accumulate extraordinarily low levels of intracellular Fe (Fig. 4B), explaining the strong induction of the Fe starvation stress response at the mRNA level (Fig. 4A). Moreover, this Fe deficiency of C. albicans mac1Δ/Δ mutants is totally reversed by Cu supplements (Fig. 4B), unlike AfMac1p control of A. fumigatus Fe transport which occurs independent of Cu status (Park et al., 2018). With C. albicans, the Fe starvation stress state of mac1Δ/Δ mutants appears secondary to defects in Cu homeostasis and the inability of these cells to populate FET oxidases with Cu.
Fe and Cu deficiency and the respiratory defect of mac1Δ/Δ mutants
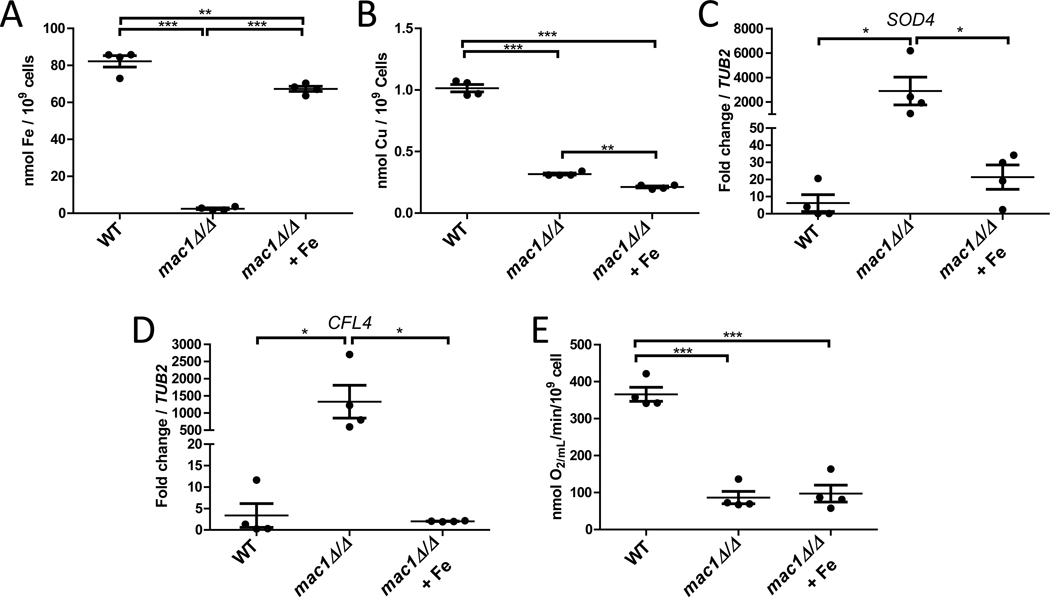
With such low Fe in mac1Δ/Δ mutants, we questioned if limited Fe bioavailability could be contributing to the respiration defect of these cells. Both Complex I and Complex II of the electron transport chain make use of Fe-S clusters (Paul et al., 2017) and Complex II, III, and IV all make use of Fe in the form of heme (Kim et al., 2012). To test the impact of low Fe on mac1Δ/Δ respiration, we sought to uncouple the Fe and Cu deficiencies of this mutant. Through Fe titrations we found that supplementation of 350 μM ferrous ammonium sulfate to the growth media restored Fe levels in the mac1Δ/Δ mutant to near WT levels (Fig. 5A), with no elevation in intracellular Cu (Fig. 5B). This restoration of intracellular Fe pools also alleviated the Fe starvation stress response of this mutant as demonstrated by reversal of the strong mac1Δ/Δ induction of the CFL4 and SOD4 gene targets of the Sef1/Sfu1p Fe regulatory system (Fig. 5C,D) (Chen et al., 2011). However, amelioration of the mac1Δ/Δ Fe deficiency had no effect on the poor respiration of these mutants (Fig. 5E), compared to the strong rescue of respiration with Cu supplements (Fig. 2C). The mac1Δ/Δ respiratory phenotype is overwhelmingly driven by deficiencies in Cu, not Fe.
Figure 5. The mac1Δ/Δ deficiency in Fe does not contribute to the mac1Δ/Δ respiratory defect.
Intracellular levels of Fe (A) or of Cu (B) were measured as in Fig. 4B and 2B, respectively. (C,D) mRNA levels of SOD4 and CFL4 was monitored by qRT-PCR in the indicated strains and values normalized to TUB2. (E) KCN-inhibitable oxygen consumption was measured as in Fig. 2C. Values are from 4 independent cultures over 2 experimental trials. Where designated, cultures were supplemented with 350 μM ferrous ammonium sulfate (“+ Fe”). Statistical significance was determined by one-way ANOVA with Tukey posttest; *p≤0.05, **p≤0.01, ***p≤0.001. The absence of a bracket indicates that comparisons are not significantly different. Among the data points, bar represents the mean with error bars showing the SEM. Strains utilized: WT, SC5314; mac1Δ/Δ, EC004.
Evidence for mac1Δ/Δ defects in Cu allocation for Fe uptake
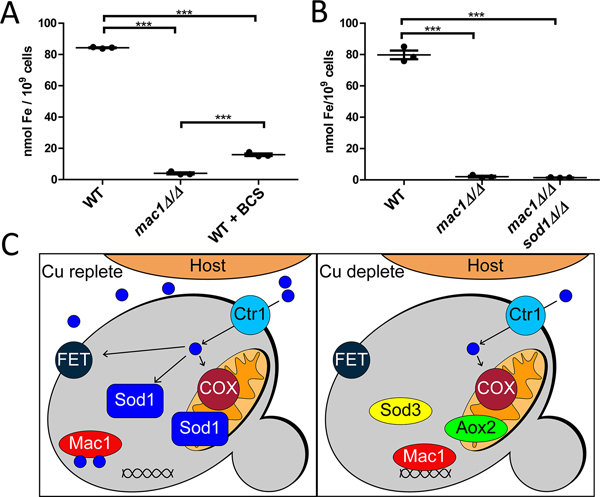
It is important to note that the Fe starvation state of mac1Δ/Δ cells cannot wholly be explained by low total Cu. The same Fe stress response is not mirrored in WT cells treated with BCS (Fig. S2A, Fig. 4A), even though these cells accumulate similarly low levels of Cu (Fig. 2B, S1A). Only a small number of the Fe transport genes were induced in BCS-WT cells, including CFL2, CFL5, FET31, and the level of induction was greatly reduced compared to that of mac1Δ/Δ cells (Fig. 4A). Just SOD4, which is not involved in Fe transport but is part of the Fe starvation regulon (Chen et al., 2011, Schatzman et al., 2020, Sorgo et al., 2013), is induced to similar levels in BCS-treated WT versus mac1Δ/Δ cells (Fig. 4A). Furthermore, BCS-treated WT cells accumulate substantially higher levels of Fe compared to mac1Δ/Δ cells (Fig. 6A). These comparisons to Cu-starved WT cells demonstrate that the severe Fe deficiency of mac1Δ/Δ cells is not simply due to low total Cu. As with COX respiration, the mac1Δ/Δ mutant appears impaired in proper allocation of Cu for Fe uptake.
Figure 6. The metal deficiencies of mac1Δ/Δ cells and a proposed model for Mac1p in Cu homeostasis.
(A,B) Intracellular Fe concentrations of the indicated strains was measured as in Fig. 4B. Statistical significance was determined by one-way ANOVA with Tukey posttest; ***p≤0.001. The absence of a bracket indicates that comparisons are not significantly different. Within the data points representing three independent cultures, wide bar represents mean with error bars showing the SEM. Strains utilized: WT, SC5314; mac1Δ/Δ, EC004 (part A) or EC006 (part B); mac1Δ/Δ sod1Δ/Δ, EC007. (C) Shown is a model for how C. albicans Mac1p regulates Cu homeostasis during infection. In the early stages of kidney infiltration (left), host Cu availability is high and there is sufficient Cu for FET multicopper oxidases for Fe uptake, for Cu/Zn Sod1p and for COX for mitochondrial respiration. At later stages (right), the host limits Cu availability to C. albicans. Mac1p is activated and induces Cu import as has been shown for numerous fungi (Woodacre et al., 2008, Marvin et al., 2004, Jungmann et al., 1993, Gross et al., 2000, Kusuya et al., 2017, Cai et al., 2019). Additionally, C. albicans Mac1p represses SOD1, and induces SOD3 and AOX2 to maintain ROS homeostasis in the cytosol and mitochondria, respectively. The repression of the major Cu consumer Sod1p serves to maintain high COX respiration in the mitochondria through a Cu sparing mechanism. C. albicans Mac1p also helps spare a limited amount of Cu for Fe uptake, through a mechanism that does not involve SOD1 repression.
Since the respiratory defect of mac1Δ/Δ cells was improved upon loss of the Sod1p Cu consumer (Fig. 3B), we tested if a deletion in SOD1 would likewise restore Fe accumulation. Significantly and unlike respiration, deletion of SOD1 had no effect on the Fe deficiency of the mac1Δ/Δ mutant (Fig. 6B). Mac1p repression of SOD1 helps spare Cu for respiration but not for Fe uptake. Other Cu consumers may be preventing Cu allocation for Fe uptake and one family of genes that are suspect are the FETs themselves. Candida has 5 annotated FETs: Fet3p, Fet31p, Fet33p, Fet34p, and Fet99p (Muzzey et al., 2013). Of these, Fet34p and Fet99p are reported to be the most important for Fe acquisition (Mamouei et al., 2017) and both of the corresponding genes are strongly induced in mac1Δ/Δ strains as part of the Fe starvation stress response (Fig. 4A). Of the FETs less critical for Fe uptake (Fet3p, Fet33p, Fet31p; (Mamouei et al., 2017)), FET31 is 100x fold induced in mac1Δ/Δ mutants, while FET3 and FET33 are not substantially changed (Fig. 4A). It is possible that Cu binding to highly abundant Fet31p may preclude proper Cu allocation to the more essential FETs for Fe uptake. Other possible Cu consumers of the cell include the putative amine oxidases Amo1p and Amo2p and although neither is induced in mac1Δ/Δ strains (Table S1), they may contribute to poor Cu availability. Additionally, we cannot exclude the possibility that Cu is sequestered in organelles such as the vacuole in the mac1Δ/Δ strain. These mechanisms for poor Cu allocation might also contribute in part to the respiratory defect of mac1Δ/Δ strains, on top of the major impact of retaining Sod1p in these Cu-starved cells.
Conclusions:
Across numerous fungal species, the Cu sensor Mac1p/Cuf1p controls cell surface uptake of Cu to ensure that intracellular Cu levels remain ample under conditions where extracellular Cu is low (Marvin et al., 2004, Woodacre et al., 2008, Li et al., 2015, Jungmann et al., 1993, Labbe et al., 1997, Garcia-Santamarina et al., 2018, Jiang et al., 2011, Ding et al., 2011, Kusuya et al., 2017, Cai et al., 2017, Park et al., 2017). Our studies demonstrate that in addition to this role in Cu uptake, Mac1p in C. albicans participates in Cu allocation and prioritizing Cu for processes such as Fe uptake and cellular respiration. This role in Cu allocation is accomplished in part through down-regulation of a major Cu consumer Sod1p and re-directing Cu towards COX for mitochondrial respiration. Previously, Mac1p control of C. albicans anti-oxidant genes SOD1, SOD3 and AOX2 was shown to maintain oxidative stress protection and ROS signaling during times of Cu limitation (Broxton & Culotta, 2016, Broxton et al., 2018). We now show this regulation of antioxidant genes has the added purpose of sparing Cu for respiration that is essential for pathogenesis of C. albicans. These findings with Mac1p may not be unique to C. albicans. C. neoformans also represses SOD1 during Cu limitation (Garcia-Santamarina et al., 2018) and the Cu may be re-purposed for other cuproenzymes essential for pathogenesis.
Experimental Procedures
Fungal strains and culture conditions
Cultures of C. albicans were maintained at 30°C in YPD (1% yeast extract, 2% peptone, 2% dextrose). Where indicated, cultures were supplemented 400 μM of the Cu(I) chelator BCS. Tests for respiration-dependent growth used non-fermentable carbon source containing YPGE medium (2% bacto-peptone, 1% yeast extract, 3% glycerol, 2% ethanol) and both YPD and YPGE plates were made with 2% bactoagar. YPD and YPGE plates supplemented with Cu contained 100 μM CuSO4. YPM media (1% yeast extract, 2% peptone, 2% maltose) was used to stimulate flippase activity. When noted, Fe was supplemented to liquid cultures as ferrous ammonium sulfate (Sigma Chemical Co.)) and Cu was added as CuSO4 (Sigma-Aldrich)
C. albicans clinical isolate SC5314 was used as the WT and parental strain from which all mutants were derived. Construction of the mac1Δ/Δ mutant by the SAT1-flipper method was as follows using primers described in Table S2: genomic DNA from strain SC5314 was used to amplify MAC1 residues −441 to −205 and +1423 to +1593. These were inserted respectively into the SacI and NotI and the XhoI and KpnI sites of the pSFS2 plasmid (Reuss et al., 2004), generating plasmid pEC-M1L. The mac1 deletion cassette from pEC-M1L was mobilized by KpnI and SacI digestion and used to transform SC5314 by electroporation. Cells were plated onto YPD containing 200 mg/mL nourseothricin sulfate (NAT) (Gold Bio), and NAT resistant colonies selected and verified for presence of the NAT cassette by PCR. Positive colonies were then grown overnight in YPM media to induce excision of NAT resistance by flippase. Cells were then plated onto 25 mg/mL NAT containing YPD media and NAT sensitive colonies were selected. The resultant mac1Δ/+ heterozygous mutant strain EC003 was verified by PCR. To delete the second allele, MAC1 sequences −205 to −1 and +1201 to +1419 were amplified by PCR and inserted into the SacI and NotI, and XhoI and KpnI sites in pSFS2 to generate the plasmid pEC-M1S. The deletion cassette from pEC-M1S was liberated with KpnI and SacI digestion and used to transform the mac1Δ/+ strain EC003. NAT resistant colonies were selected and NAT resistance excised as above. Deletion of the second MAC1 allele was verified by PCR, resulting in the mac1Δ/Δ strain EC004.
A single copy of MAC1 was introduced in the mac1Δ/mac1Δ strain EC004 as follows: MAC1 genomic sequences from −205 to +1419 were amplified and inserted into pSFS2 at SacI and NotI sites. MAC1 residues +1209 to 1419 were inserted into XhoI and KpnI sites of this plasmid to generate the pEC-M1R plasmid, which was then linearized with SacI and KpnI and transformed into the mac1Δ/Δ strain EC004, generating the mac1Δ/Δ::MAC1 re-integrant strain EC005.
A CRISPR protocol optimized for C. albicans was used as previously described (Nguyen et al., 2017) to generate the mac1Δ/Δ sod1Δ/Δ and a mac1Δ/Δ control strain using oligonucleotides listed in Table S3. Homozygous null mutations were introduced using a donor DNA oligo 100 bp in length composed of residues −50 to −1 upstream and 50 base pairs directly downstream of the coding regions of MAC1 and SOD1, respectively. Guide RNAs to MAC1 and SOD1 were designed using Benching Software. Both SOD1 and MAC1 were simultaneously deleted in C. albicans strain SC5314 to generate strain EC007 and the mac1Δ/Δ single homozygous mutant strain EC006 was generated similarly. Strains were confirmed by PCR and DNA sequencing.
Fungal cells were photographed using dark field microscopy using a Nikon Infinity 1 microscope at 40x magnification.
Murine Virulence Studies
Murine studies were carried out according to the National Institutes of Health guidelines for the ethical treatment of animals. This protocol was approved by the Institutional Animal Care and Uses Committee of the Johns Hopkins University medical institutions, protocol number M013M264. Nine-week old BALB/c female mice per strain in groups of ten were infected with 2×105 C. albicans cells in 100 μL by lateral tail injection as previously described (Conti et al., 2014). Female mice were used as no difference between sexes has been noted in regards to the Cu response seen in the kidney during infection (Besold et al., 2018, Li et al., 2015, Culbertson et al., 2020). The fungal cells for infection were obtained by growth overnight in YPD to OD600 ~15; cells were harvested by centrifugation, washed twice in sterile 1x phosphate buffered saline (PBS), enumerated on a hemocytometer and diluted to 2×106 cells mL−1 in PBS. Mouse survival following injection was monitored daily up to 30 days.
Metal measurements
For Cu analysis, cells were grown in YPD to OD600 of ≈2.0. 10–20 OD600 cell units were harvested by centrifugation and washed twice with 10 mM Tris, 1 mM EDTA, pH 8, and twice with MilliQ deionized water and cell recovery determined by measuring OD600. Cells were digested with 200 μL 10% nitric acid (Fisher Chemical) at 100°C, diluted to a final concentration of 2% nitric acid in MilliQ water and then Cu content was measured using an AAnalyst graphite furnace atomic absorption spectrometer (AAS) (PerkinElmer). Metal content was normalized to cell number.
For Fe measurements, samples were subjected to a bathophenanthroline disulfonate (BPS) based assay as previously described (Tamarit et al., 2006). Cells for Fe analysis were prepared in nitric acid as described above for AAS. A plate-based version of the assay was adopted in which reactions contained 100 μL of standard or cell sample, 75 μL of water, 40 μL of 38 mg ml−1 sodium ascorbate, and 32 μL of a 1/3 saturated ammonium acetate solution. A first measurement of absorbance at 535 and 680 nm was then measured on an Eon (Biotek) plate reader. After the first spectrophotometric readings, 3 μL of 34 mg mL−1 BPS was added to each well. A second set of absorbance measurements at 535 and 680 nm was then measured. The BPS signal was determined as X = ((A535-A680)sample+BPS – (A535-A680)sample-BPS) – ((A535-A680)blank+BPS – (A535-A680)blank-BPS).
Oxygen Consumption Assays for COX respiration
Oxygen consumption measurements were conducted on whole cells similarly to published protocols (Li et al., 2011, Broxton & Culotta, 2016) with modifications. Cells were grown in YPD to an OD600 of ≈2.0, harvested by centrifugation and resuspended to an OD600 of 5.0 and allowed to grow for 1 hour in fresh YPD at 30°C. 500 μL of the 5 mL culture were added to 1 mL of YPD in a Clark-type electrode (Hanstech Oxythem Plus) in a magnetically stirred, thermostatically controlled 1.5 mL chamber at 30°C. Oxygen saturation was measured over the course of 3–5 minutes and the change in oxygen saturation over time was used to determine the oxygen consumption rate. To validate oxygen consumption dependent on COX-mitochondrial respiration, 10 mM potassium cyanide (KCN) was added to inhibit COX respiration as described (Li et al., 2011). For all respiration experiments 3 cultures for each strain served as the biological replicates and for each biological replicate 2 technical replicates of oxygen consumption were averaged.
Analyses of SOD protein and enzyme activity
For immunoblots and SOD activity gels, cells were grown to an OD600 of ≈2 in 10 mL YPD. 10 OD600 cell units were harvested, washed in water and resuspended in 100 μL of a lysis buffer containing 5 mM EDTA, 5 mM EGTA, 50 mM NACL, 10% glycerol, and 0.1% Triton X-100 in 10 mM sodium phosphate, pH 7.8. An equal volume of 0.5 mm zirconium oxide beads (Research Products International) was added and the samples were vortexed three times for a minute and a half at 4°C. Samples were then centrifuged for 10 minutes at 14,000xg. Supernatant was removed and protein concentration was determined via Bradford method. The resultant protein lysates were used for immunoblot analysis and SOD activity.
Immunoblot analysis for Sod1p and Sod3p followed published procedures (Gleason et al., 2014). Briefly 10–15 μg of the samples were subjected to denaturing gel electrophoresis on 4–12% Bis-Tris acrylamide gels (Thermo Fisher). Proteins were then transferred to a PVDF membrane and blocked in 2% non-fat milk (VWR Life Science). Blots were probed with anti-SOD1(Jensen & Culotta, 2005) at a 1:10000 dilution and anti-SOD3 (Gleason et al., 2014) at 1:5000. Primary antibody incubation was followed by incubation with secondary goat anti-rabbit IgG Alexa Flour 680 antibody at 1:10,000 (Thermofisher Scientific). Blots were imaged on an Odyssey infrared imaging system (LI-COR Biosciences) at 700 nm.
For analysis of SOD activity, lysates were run on native non-reducing 10% Tris-glycine gels at 50 mA for 90 minutes at 4°C. Gels were then stained with 35 mL of a potassium phosphate buffered solution containing Nitro Blue Tetrazolium (Sigma-Aldrich), riboflavin (Acros Organics) and 35 μL of TEMED (Invitrogen) as previously published (Gleason et al., 2014). Gels were incubated in this solution for 1 hour in the dark with gentle shaking. Gels were then placed in dH2O, exposed to light and photographed.
RNA analysis by qRT-PCR and RNA-seq
To prepare RNA for qRT-PCR or RNA-seq, triplicate cultures were grown 16 hours to an OD600 of ≈ 2.0 in YPD. RNA was extracted from at least five OD600 cell units via an acid-phenol protocol as previously described (Collart & Oliviero, 2001). Briefly cells were lysed for an hour at 65°C with vortexing every 5 minutes in 400 μL of acid phenol pH 4.5 (Ambion AM9720), 350 μL of 3M sodium acetate pH 5.5 (Thermo Fisher Scientific), and 1% SDS. Samples were subjected to three organic extractions with acid-phenol, precipitated by ethanol and nucleic acids treated with DNAse by Rapidout (ThermoFisher Scientific K2981). For qRT-PCR, cDNA was synthesized using a RevertAid First Strand cDNA (ThermoFIsher Scientific K1622). cDNA samples were diluted 1:10 in DEPC-treated water before qRT-PCR analysis with PowerUp SYBR Green Master Mix (ThermoFisher Scientific). qRT-PCR was performed on a QuantStudio 3 (Applied Biosystems) instrument. Cycle threshold values (Ct values) were normalized to TUB2. Relative expression was calculated using the dCT method. Amplicons of ~200 bp were obtained from the primers for TUB2, CTR1, CFL4, and SOD4 shown in the Table S4. For RNA-seq, RNA samples were processed by Novogene Corporation Inc. for paired-end sequencing on an Ilumina HiSeq 2500. Reads were aligned to the C. albicans reference genome (SC5314) using TopHat2 (Kim et al., 2013). Aligned reads were then used to generate read counts for each gene and the DE-seq package from Bioconductor was used for statistical analysis of differential gene expression (Anders & Huber, 2010). The RNA-seq data that support the findings of this study will be deposited to the NCBI sequence read archive (SRA) under BioProject accession no. PRJNA649282 and will be openly available.
Software and Statistics
Differences were considered statically significantly if comparisons for P values were ≤ 0.05 using a one-way analysis of variance (ANOVA) with a Tukey posttest as determined by Graphpad Prism 7. Mouse survival was analyzed using a log rank test (Mantel Cox) to query any statistical differences. A gene was considered differentially expressed if its FDR for differential expression was <0.05. For GO analysis we used the Candida Genome Data Base’s GO Term Finder and report GO terms with an FDR of less than 25%.
Supplementary Material
Acknowledgements
We would like to thank Dr. Angelique Besold and Danielle Bouchard for helpful discussions. This work was supported by NIH grants RO1 AI119949 (VCC and BPC), RO1 GM136644 (VCC), U19 AI110820 (VMB), and F31 DK111114 (EMC).
Footnotes
Conflicts of Interest
There are no conflicts of interest to declare.
References:
- Anders S, and Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Ito-Kuwa S, Nakamura Y, and Masuhara T. (1990) Comparative pathogenicity of a wild-type strain and respiratory mutants of Candida albicans in mice. Zentralbl Bakteriol 273: 332–343. [DOI] [PubMed] [Google Scholar]
- Bambach A, Fernandes MP, Ghosh A, Kruppa M, Alex D, Li D, Fonzi WA, Chauhan N, Sun N, Agrellos OA, Vercesi AE, Rolfes RJ, and Calderone R. (2009) Goa1p of Candida albicans localizes to the mitochondria during stress and is required for mitochondrial function and virulence. Eukaryot Cell 8: 1706–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JM, Kauffman SJ, Hauser M, Huang L, Lin M, Sillaots S, Jiang B, Xu D, and Roemer T. (2010) Pathway analysis of Candida albicans survival and virulence determinants in a murine infection model. Proc Natl Acad Sci U S A 107: 22044–22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, and Culotta VC (2018) Role of Calprotectin in Withholding Zinc and Copper from Candida albicans. Infect Immun 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxton CN, and Culotta VC (2016) An Adaptation to Low Copper in Candida albicans Involving SOD Enzymes and the Alternative Oxidase. PLoS One 11: e0168400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxton CN, He B, Bruno VM, and Culotta VC (2018) A role for Candida albicans superoxide dismutase enzymes in glucose signaling. Biochem Biophys Res Commun 495: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Du W, Liu L, Pan D, and Lu L. (2019) Molecular Characteristics of the Conserved Aspergillus nidulans Transcription Factor Mac1 and Its Functions in Response to Copper Starvation. mSphere 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Du W, Zeng Q, Long N, Dai C, and Lu L. (2017) Cu-sensing transcription factor Mac1 coordinates with the Ctr transporter family to regulate Cu acquisition and virulence in Aspergillus fumigatus. Fungal Genet Biol 107: 31–43. [DOI] [PubMed] [Google Scholar]
- Chen C, Pande K, French SD, Tuch BB, and Noble SM (2011) An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10: 118–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, and Miethke M. (2010) Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. J Bacteriol 192: 2512–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, and Oliviero S. (2001) Preparation of yeast RNA. Curr Protoc Mol Biol Chapter 13: Unit13 12. [DOI] [PubMed] [Google Scholar]
- Conti HR, Huppler AR, Whibley N, and Gaffen SL (2014) Animal models for candidiasis. Curr Protoc Immunol 105: 19 16 11–19 16 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson EM, Khan AA, Muchenditsi A, Lutsenko S, Sullivan DJ, Petris MJ, Cormack BP, and Culotta VC (2020) Changes in mammalian copper homeostasis during microbial infection. Metallomics 12: 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Howard WR, and Liu XF (1994) CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J Biol Chem 269: 25295–25302. [PubMed] [Google Scholar]
- Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, and Thiele DJ (2011) The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol 81: 1560–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa RA, and Thiele DJ (2011) Copper: an essential metal in biology. Curr Biol 21: R877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sanchez S, Mavor AL, Russell CL, Argimon S, Dennison P, Enjalbert B, and Brown AJ (2005) Global roles of Ssn6 in Tup1- and Nrg1-dependent gene regulation in the fungal pathogen, Candida albicans. Mol Biol Cell 16: 2913–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Santamarina S, Festa RA, Smith AD, Yu CH, Probst C, Ding C, Homer CM, Yin J, Noonan JP, Madhani H, Perfect JR, and Thiele DJ (2018) Genome-wide analysis of the regulation of Cu metabolism in Cryptococcus neoformans. Mol Microbiol 108: 473–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason JE, Li CX, Odeh HM, and Culotta VC (2014) Species-specific activation of Cu/Zn SOD by its CCS copper chaperone in the pathogenic yeast Candida albicans. J Biol Inorg Chem 19: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Kelleher M, Iyer VR, Brown PO, and Winge DR (2000) Identification of the copper regulon in Saccharomyces cerevisiae by DNA microarrays. J Biol Chem 275: 32310–32316. [DOI] [PubMed] [Google Scholar]
- Hippmann AA, Schuback N, Moon KM, McCrow JP, Allen AE, Foster LJ, Green BR, and Maldonado MT (2017) Contrasting effects of copper limitation on the photosynthetic apparatus in two strains of the open ocean diatom Thalassiosira oceanica. PLoS One 12: e0181753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GH, Nie XY, and Chen JY (2006) CaMac1, a Candida albicans copper ion-sensing transcription factor, promotes filamentous and invasive growth in Saccharomyces cerevisiae. Acta Biochim Biophys Sin (Shanghai) 38: 213–217. [DOI] [PubMed] [Google Scholar]
- Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, and Kang SO (2002) Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology-Sgm 148: 3705–3713. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Rognoni F, van Duijn W, Verspaget HW, Haasdijk ED, and Holstege JC (2001) CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol 102: 293–305. [DOI] [PubMed] [Google Scholar]
- Jensen LT, and Culotta VC (2005) Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J Biol Chem 280: 41373–41379. [DOI] [PubMed] [Google Scholar]
- Jiang N, Liu X, Yang J, Li Z, Pan J, and Zhu X. (2011) Regulation of copper homeostasis by Cuf1 associates with its subcellular localization in the pathogenic yeast Cryptococcus neoformans H99. FEMS Yeast Res 11: 440–448. [DOI] [PubMed] [Google Scholar]
- Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, Shaw DE, Tonks NK, Mazar AP, and Donate F. (2008) Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci U S A 105: 7147–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J, Reins HA, Lee J, Romeo A, Hassett R, Kosman D, and Jentsch S. (1993) MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J 12: 5051–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G, Bird A, and Winge DR (2005) Independent metalloregulation of Ace1 and Mac1 in Saccharomyces cerevisiae. Eukaryot Cell 4: 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiri I, Tebbji F, and Sellam A. (2020) Transcriptome Analysis Uncovers a Link Between Copper Metabolism, and Both Fungal Fitness and Antifungal Sensitivity in the Opportunistic Yeast Candida albicans. Front Microbiol 11: 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, and Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Khalimonchuk O, Smith PM, and Winge DR (2012) Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim Biophys Acta 1823: 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Gallaher SD, Urzica EI, Nakamoto SS, Strenkert D, Tottey S, Mason AZ, and Merchant SS (2015) Copper economy in Chlamydomonas: prioritized allocation and reallocation of copper to respiration vs. photosynthesis. Proc Natl Acad Sci U S A 112: 2644–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuya Y, Hagiwara D, Sakai K, Yaguchi T, Gonoi T, and Takahashi H. (2017) Transcription factor Afmac1 controls copper import machinery in Aspergillus fumigatus. Curr Genet 63: 777–789. [DOI] [PubMed] [Google Scholar]
- Labbe S, Zhu Z, and Thiele DJ (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem 272: 15951–15958. [DOI] [PubMed] [Google Scholar]
- Lamarre C, LeMay JD, Deslauriers N, and Bourbonnais Y. (2001) Candida albicans expresses an unusual cytoplasmic manganese-containing superoxide dismutase (SOD3 gene product) upon the entry and during the stationary phase. J Biol Chem 276: 43784–43791. [DOI] [PubMed] [Google Scholar]
- Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, and Culotta VC (2015) Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen H, Florentino A, Alex D, Sikorski P, Fonzi WA, and Calderone R. (2011) Enzymatic dysfunction of mitochondrial complex I of the Candida albicans goa1 mutant is associated with increased reactive oxidants and cell death. Eukaryot Cell 10: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev SI, and Fridovich I. (2002) The Haber-Weiss cycle -- 70 years later: an alternative view. Redox Rep 7: 55–57; author reply 59–60. [DOI] [PubMed] [Google Scholar]
- MacCallum DM, and Odds FC (2005) Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48: 151–161. [DOI] [PubMed] [Google Scholar]
- Mackie J, Szabo EK, Urgast DS, Ballou ER, Childers DS, MacCallum DM, Feldmann J, and Brown AJ (2016) Host-Imposed Copper Poisoning Impacts Fungal Micronutrient Acquisition during Systemic Candida albicans Infections. PLoS One 11: e0158683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamouei Z, Zeng G, Wang YM, and Wang Y. (2017) Candida albicans possess a highly versatile and dynamic high-affinity iron transport system important for its commensal-pathogenic lifestyle. Mol Microbiol 106: 986–998. [DOI] [PubMed] [Google Scholar]
- Marvin ME, Mason RP, and Cashmore AM (2004) The CaCTR1 gene is required for high-affinity iron uptake and is transcriptionally controlled by a copper-sensing transactivator encoded by CaMAC1. Microbiology 150: 2197–2208. [DOI] [PubMed] [Google Scholar]
- Merchant S, and Bogorad L. (1986) Regulation by copper of the expression of plastocyanin and cytochrome c552 in Chlamydomonas reinhardi. Mol Cell Biol 6: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S, Hill K, and Howe G. (1991) Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J 10: 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montllor-Albalate C, Colin AE, Chandrasekharan B, Bolaji N, Andersen JL, Wayne Outten F, and Reddi AR (2019) Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox Biol 21: 101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzey D, Schwartz K, Weissman JS, and Sherlock G. (2013) Assembly of a phased diploid Candida albicans genome facilitates allele-specific measurements and provides a simple model for repeat and indel structure. Genome Biol 14: R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Quail MMF, and Hernday AD (2017) An Efficient, Rapid, and Recyclable System for CRISPR-Mediated Genome Editing in Candida albicans. mSphere 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado-Matsumoto A, and Fridovich I. (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver Cu,Zn-SOD in mitochondria. j Biol Chem 276: 38388–38393. [DOI] [PubMed] [Google Scholar]
- Park YS, Kang S, Seo H, and Yun CW (2018) A copper transcription factor, AfMac1, regulates both iron and copper homeostasis in the opportunistic fungal pathogen Aspergillus fumigatus. Biochem J 475: 2831–2845. [DOI] [PubMed] [Google Scholar]
- Park YS, Kim TH, and Yun CW (2017) Functional characterization of the copper transcription factor AfMac1 from Aspergillus fumigatus. Biochem J 474: 2365–2378. [DOI] [PubMed] [Google Scholar]
- Paul BT, Manz DH, Torti FM, and Torti SV (2017) Mitochondria and Iron: current questions. Expert Rev Hematol 10: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, and O’Halloran TV (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284: 805–808. [DOI] [PubMed] [Google Scholar]
- Reddi AR, and Culotta VC (2013) SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 152: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss O, Vik A, Kolter R, and Morschhauser J. (2004) The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341: 119–127. [DOI] [PubMed] [Google Scholar]
- Schatzman SS, Peterson RL, Teka M, He B, Cabelli DE, Cormack BP, and Culotta VC (2020) Copper-only superoxide dismutase enzymes and iron starvation stress in Candida fungal pathogens. J Biol Chem 295: 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, and Valentine JS (2014) Superoxide dismutases and superoxide reductases. Chem Rev 114: 3854–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Prasad HK, Sinha I, Agarwal N, and Natarajan K. (2011) Cap2-HAP complex is a critical transcriptional regulator that has dual but contrasting roles in regulation of iron homeostasis in Candida albicans. J Biol Chem 286: 25154–25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgo AG, Brul S, de Koster CG, de Koning LJ, and Klis FM (2013) Iron restriction-induced adaptations in the wall proteome of Candida albicans. Microbiology 159: 1673–1682. [DOI] [PubMed] [Google Scholar]
- Sturtz LA, Diekert K, Jensen LT, Lill R, and Culotta VC (2001) A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem 276: 38084–38089. [DOI] [PubMed] [Google Scholar]
- Tamarit J, Irazusta V, Moreno-Cermeno A, and Ros J. (2006) Colorimetric assay for the quantitation of iron in yeast. Anal Biochem 351: 149–151. [DOI] [PubMed] [Google Scholar]
- Tan G, Cheng Z, Pang Y, Landry AP, Li J, Lu J, and Ding H. (2014) Copper binding in IscA inhibits iron-sulphur cluster assembly in Escherichia coli. Mol Microbiol 93: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele DJ (1988) ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol 8: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski ML, and Thiele DJ (2009) New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem 284: 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl MA, Biery M, Craig N, and Johnson AD (2003) Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C.albicans. EMBO J 22: 2668–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SR, Hacham M, Hu G, Zhu X, Park YD, Shin S, Panepinto J, Valyi-Nagy T, Beam C, Husain S, Singh N, and Williamson PR (2007) Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest 117: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SR, Park YD, Raja M, Qiu J, Hammoud DA, O’Halloran TV, and Williamson PR (2012) Role of CTR4 in the Virulence of Cryptococcus neoformans. Mbio 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger RA, and Fridovich I. (1973) Mitochondrial superoxide dismutase site of synthesis and intramitochondrial localization. J Biol Chem 248: 4793–4796. [PubMed] [Google Scholar]
- Woodacre A, Mason RP, Jeeves RE, and Cashmore AM (2008) Copper-dependent transcriptional regulation by Candida albicans Mac1p. Microbiology 154: 1502–1512. [DOI] [PubMed] [Google Scholar]
- Ziegler L, Terzulli A, Gaur R, McCarthy R, and Kosman DJ (2011) Functional characterization of the ferroxidase, permease high-affinity iron transport complex from Candida albicans. Mol Microbiol 81: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.