Abstract
Introduction:
Aicardi Goutières Syndrome (AGS) is a genetic interferonopathy characterized by early onset of severe neurologic injury with intracranial calcifications, leukoencephalopathy, and systemic inflammation. Increasingly, a spectrum of neurologic dysfunction and presentation beyond the infantile period is being recognized in AGS. The aim of this study was to characterize late-infantile and juvenile onset AGS.
Methods:
We conducted a multi-institution, retrospective review of individuals with AGS who presented over 1 year old, including medical history, imaging characteristics and suspected diagnoses at presentation.
Results:
Thirty-four individuals were identified, all with pathogenic variants in RNASEH2B, SAMHD1, ADAR1, or IFIH1. Most individuals had a history of developmental delay and/or systemic symptoms, such as sterile pyrexias and chilblains, followed by a prodromal period associated with increasing symptoms. This was followed by an abrupt onset of neurologic decline (fulminant phase), with a median onset at 1.33 years (range 1.00–17.68 years). Most individuals presented with a change in gross motor skills (97.0%), typically with increased tone (78.8%). Leukodystrophy was the most common MRI finding (40.0%). Calcifications were less common (12.9%).
Conclusions:
This is the first study to characterize presentation of late-infantile and juvenile onset AGS and its phenotypic spectrum. Late-onset AGS can present insidiously and lacks classic clinical and neuroimaging findings. Signs of early systemic dysfunction prior to fulminant disease onset and loss of motor symptoms were common. We strongly recommend genetic testing when there is concern for sustained inflammation of unknown origins or changes in motor skills in children more than one year of age.
Keywords: Aicardi Goutières Syndrome, leukodystrophy, RNASEH2B, ADAR1, IFIH1, SAMHD1, type I interferonopathy, ADEM, spastic diplegia
Introduction
Aicardi Goutières Syndrome (AGS) is a rare genetic interferonopathy that results in heterogeneous neurologic injury. The neonatal (likely in utero) form is a mimic of congenital viral (TORCH) infections and is commonly linked with the TREX1 genotype [1–5]. Early-infantile AGS typically presents weeks to months after birth, and is typically characterized by an acute encephalitic phase, followed by stabilization of neurologic function [4]. As the recognition of AGS has improved, a spectrum of neurologic dysfunction has been described beyond the early infantile period [1, 3, 6, 7]. Late-onset AGS, or ‘atypical’ AGS, poses a particular diagnostic challenge because it appears to be the most heterogeneous [8].
AGS is associated with by systemic auto-inflammation secondary to pathologic upregulation of the type I interferon-mediated immune system [9, 10]. There are currently 7 genes linked to AGS: TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1. These genes are involved in intracellular nucleic acid metabolism. TREX1, RNASEH2A/B/C, and SAMHD1 regulate nucleic acid maintenance and repair [11–15]. Pathogenic variants in these genes result in an accumulation of endogenous retro-elements, which triggers RNA/DNA sensing pathways and IFN activation [16–18]. ADAR1 and IFIH1 are part of a nucleic acid sensing pathway [19–21]. Regardless of the specific mechanism, all forms of AGS result in elevated interferon levels in the blood and cerebrospinal fluid, as measured indirectly by interferon signaling gene expression scores [4, 10, 22, 23]. Despite the common mechanistic pathway, outcomes and age at onset can be variable [1, 24, 25].
Neuroimaging plays a critical role in facilitating the diagnosis of AGS, although the incidence of individual features differs by the subpopulation studied. Brain calcifications, cerebral atrophy, and leukodystrophy are the most common findings [26]. Some gene-specific patterns have been described: SAMHD1-related intracerebral large vessel disease [7, 27], ADAR1-related bilateral striatal necrosis [9, 22], and RNASH2B-associated porencephalic cysts, cerebellar hypoplasia, and globus pallidus iron accumulation [28]. Notably, neuroimaging findings can change, and even improve, over time [29].
Later onset AGS is poorly understood, having been primarily described in case reports. Some individuals with ADAR1-, IFIH1- and RNASEH2B-related disease present with non-syndromic spastic paraplegia [4, 30]. Other cases of ADAR1-related AGS manifest with severe, isolated dystonia [4, 9]. One individual with IFIH1-associated AGS presented with posturing, rigidity, and exaggerated startle [3]. These atypical cases can have variable clinical courses after presentation. Some AGS individuals do not experience developmental or cognitive regression with disease onset [3, 4]. These reports reveal the diversity of AGS, and more research needed to characterize the full phenotypic spectrum.
The aim of this study was to identify common misdiagnoses of late-onset AGS and to characterize presenting signs and symptoms within a retrospective natural history study. Our aim was to provide clinicians with information to appropriately consider AGS during initial evaluation of children with neurologic regression. We hypothesized that there are particular clinical or neuroradiographic characteristics that providers can use to appropriately evaluate individuals for AGS.
Methods
Subject Ascertainment and Enrollment
Individuals were identified through the Myelin Disorders Bioregistry Project, an arm of the Global Leukodystrophy Initiative Clinical Trial Network (GLIA-CTN) from several institutions (Children’s Hospital of Philadelphia, Spedali Civili of Brescia, and Istituto di Ricerca Clinica C. Mondino). The study was approved by the Institutional Review Board. AGS was defined as a combination of neurologic disability with a pathogenic/likely pathogenic variant in an AGS-related gene. Individuals without a known genetic diagnosis or with insufficient clinical diagnostic information were excluded. Individuals diagnosed with spastic paraparesis and other subforms of AGS were included. From total of 231 individuals, we identified 34 individuals from 32 families presenting after the age of twelve months.
Retrospective history collection
Genotype, ages, prodromal history, fulminant presenting symptoms, MRI features, and initial differential diagnoses were collected through retrospective chart review. Charts with insufficient details and MRIs obtained greater than 1 year from the initial symptoms were excluded from analysis. Pathology-targeting pharmacologic treatments, such as immunomodulatory agents, and responses to treatment were noted. Age at diagnosis was defined as the date that genetic confirmation was obtained.
Early AGS-related symptomatology was divided into three categories: early phase, prodrome, and fulminant presentation. The early phase was defined as non-specific signs of abnormal growth or development in an otherwise healthy child. This category included isolated or rare inflammatory symptoms. The prodrome was defined as an increased burden of recurrent or persistent symptoms associated with inflammation prior to fulminant presentation. Systemic symptoms were noted and included fevers, fatigue, irritability/colic, myalgias/fatigue and chilblains. Fulminant symptoms were defined as new observable neurologic deficits.
The first magnetic resonance imaging (MRI) results obtained within the first two years of disease were included for review (n=31). Original MR images for 13 individuals were reviewed by a pediatric neuroradiologist, and otherwise the original radiology reports were used. One individual had MR images obtained prior to symptom onset for unrelated reasons.
Statistical analysis
Analyses were performed in GraphPad Prism 8. Demographic, clinical and genetic features were described with descriptive statistics.
Results
Cohort Features
A total of 34 individuals (17 males) were identified from our international cohort. This included individuals with variants in RNASEH2B (n=14), SAMHD1 (n=5), ADAR1 (n=10), and IFIH1 (n=5) (Table 1). Twenty individuals were diagnosed by targeted genetic panel (58.8%) and fourteen by exome sequencing (41.2%).
Table 1:
Atypical AGS cohort genotypes
| Genotype | Nucleotide #1 | Protein #1 | Inheritance | Nucleotide #2 | Protein #2 | Inheritance | Method of diagnosis |
|---|---|---|---|---|---|---|---|
| ADAR1 | c.3019G<A | p.Gly1007Arg | de novo | ES | |||
| c.3019G<A | p.Gly1007Arg | de novo | ES | ||||
| c.982C<T | p.Arg328* | UK | c.577C<G | p.Pro193Ala | paternal | ES | |
| c.577C<G | p.Pro193Ala | maternal | c.3020-3C<G | paternal | ES | ||
| c.3019G<A | p.Gly1007Arg | UK | Targeted/panel | ||||
| c.3443+1G<C | maternal | c.577C<G | p.Pro193Ala | paternal | ES | ||
| c.3019G<C | p.Gly1007Arg | UK | Targeted/panel | ||||
| c.577C<G | p.Pro193Ala | maternal | c.1076_1080del | paternal | Targeted/panel | ||
| c.3019G<A | p.Gly1007Arg | de novo | Targeted/panel | ||||
| c.3463C<T | p.Arg1155Trp | maternal | c.577C<G | p.Pro193Ala | paternal | ES | |
| IFIH1 | c.2336G<A | p.Arg779His | paternal | Targeted/panel | |||
| c.2336G<A | p.Arg779His | paternal | ES | ||||
| c.1165G<A | p.Gly389Arg | de novo | ES | ||||
| c.992C<G | p.Thr331Arg | de novo | Targeted/panel | ||||
| c.2335C<T | p.Arg779Cys | UK | Targeted/panel | ||||
| RNASEH2B | c.529G<A | p.Ala177Thr | maternal | c.529G<A | p.Ala177Thr | paternal | ES |
| c.529G<A | p.Ala177Thr | UK | c.132T<A | p.Cys44* | paternal | Targeted/panel | |
| c.554T<G | p.Val185Gly | c.554T<G | p.Val185Gly | Targeted/panel | |||
| c.529G<A | p.Ala177Thr | UK | c.529G<A | p.Ala177Thr | UK | ES | |
| c.526G<A | p.Ala177Thr | maternal | c.526G<A | p.Ala177Thr | paternal | ES | |
| c.529G<A | p.Ala177Thr | UK | c.529G<A | p.Ala177Thr | UK | ES | |
| c.529G<A | p.Ala177Thr | maternal | c.510+1G<A | paternal | ES | ||
| c.529G<A | p.Ala177Thr | maternal | c.510+1G<A | paternal | ES | ||
| c.529G<A | p.Ala177Thr | UK | c.529G<A | p.Ala177Thr | UK | Targeted/panel | |
| c.529G<A | p.Ala177Thr | UK | c.529G<A | p.Ala177Thr | UK | Targeted/panel | |
| c.529G<A | p.Ala177Thr | maternal | c.529G<A | p.Ala177Thr | paternal | Targeted/panel | |
| c.529G<A | p.Ala177Thr | maternal | c.529G<A | p.Ala177Thr | paternal | Targeted/panel | |
| c.253C<G | p.Leu85Val | maternal | c.65-13G<Ar.65-11_65-1ins | p.Glu22Valfs*5 | paternal | Targeted/panel | |
| c.529G<A | p.Ala177Thr | UK | c.529G<A | p.Ala177Thr | UK | Targeted/panel | |
| SAMHD1 | Exon 1 deletion | UK | Exon 1 deletion | UK | Targeted/panel | ||
| Exon 1 deletion | maternal | Exon 1 deletion | paternal | Targeted/panel | |||
| c.490C<T | p.Arg164* | UK | Deletion (Exons 10–13) | UK | Targeted/panel | ||
| Exon 1 deletion | maternal | Exon 1 deletion | paternal | Targeted/panel | |||
| Exon 1 deletion | UK | Exon 1 deletion | UK | Targeted/panel |
UK: unknown; ES: exome sequencing
Clinical Presentation of Atypical AGS
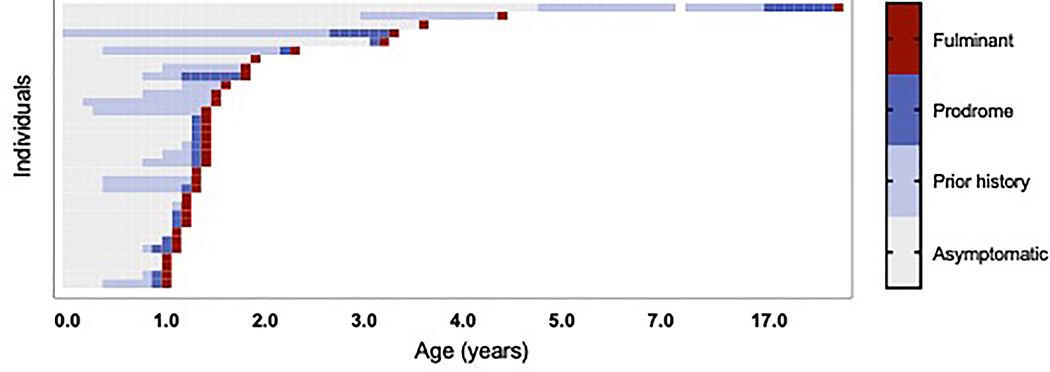
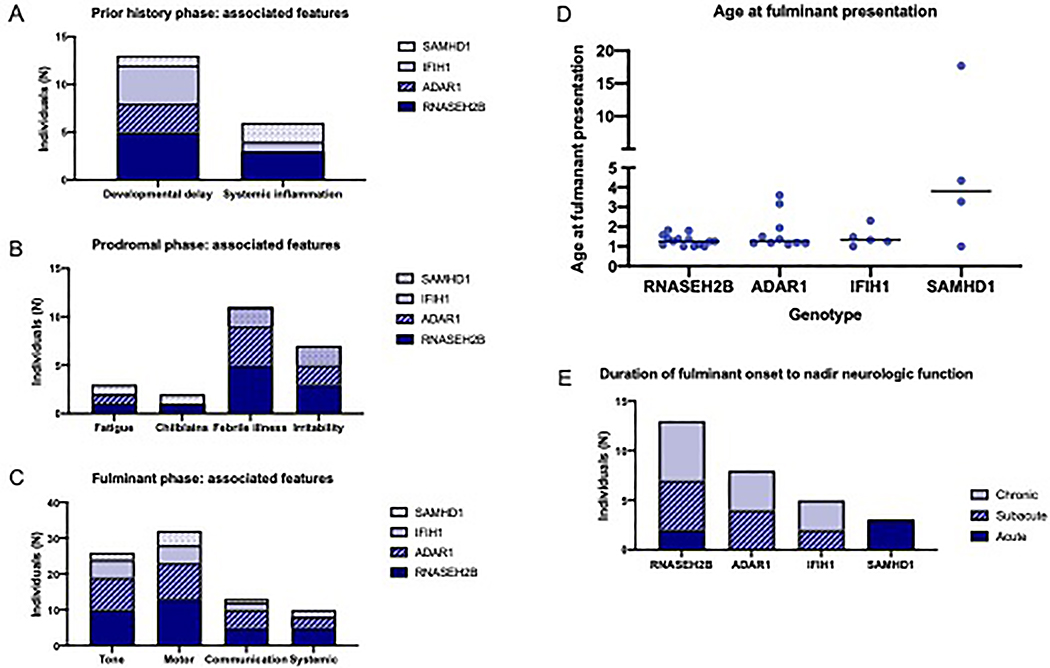
Based on clinical experience, early AGS-related symptomatology was divided into three categories: early phase, prodrome, and fulminant presentation (Figure 1). The majority of individuals with atypical AGS had a history of AGS-related complications prior to the onset of disease (n=20 of 29 with available histories, 69.0%%), beginning between birth and 4.76 years of age (median 0.81 years). Common features during this early phase included developmental delay (n=13/29, 44.8%), often accompanied by systemic signs or symptoms of inflammation (n=6/29, 20.7%), including sterile pyrexia, unexplained hypothermia, chronic pain, persistent irritability, or chilblains (Figure 2A).
Figure 1.
Progression of disease in atypical AGS cases is characterized by 3 phases: early phase, prodrome, and fulminant presentation. Each individual (n=33 with sufficient data) is represented by a row, with each phase of the disease color coded with age in years on the x-axis. Normal development with no systemic signs or symptoms of inflammation represented by grey boxes, early phase is represented by light blue; prodromal phase is represented by blue; the onset of fulminant symptoms is represented by a red box.
Figure 2.
Common features of the phases of atypical AGS by genotype. The most common clinical features during each phase were noted by genotype in non-exclusive categories. (A) In the early phase, developmental delay and systemic inflammation were most commonly noted; (B) in the prodrome phase, fatigue, irritability, sterile pyrexias (febrile illnesses), and chilblains were noted; while in the fulminant phase (C), abnormalities in tone, motor skills, communication, and systemic signs or symptoms were most common. (D) The age at fulminant presentation is presented by genotypic cohort. (E) The time to neurologic nadir (maximum symptoms) was categorized as acute (within a week), subacute (1 week to 1 month), or chronic (more than 1 month).
After the early phase, most individuals with atypical AGS demonstrated increasing features of inflammation during a “prodromal” period (n=18/25 individuals with available clinical information, 72.0%) (Figure 2B). The median age of onset of prodromal symptoms was 1.25 years (range 0.83–17.00 years). Systemic prodromal features included febrile illness (n=11/25, which included infection-related episodes in two cases), irritability (n=7), fatigue (n=3), chilblains (n=2), and myositis (n=1).
Following a period of worsening prodromal symptoms, 33 of 34 individuals with atypical AGS experienced a fulminant period of neurologic decline with a sufficiently detailed course for analysis. The median age of fulminant disease onset was 1.33 years (range 1.00–17.68 years) (Figure 2D). At the time of disease onset, most individuals (n=32 of 33, 97.0%) presented with changes in gross motor function, accompanied by a change in tone in 26 individuals (78.8%). Twenty-one individuals were noted to have new onset of spasticity, eight had dystonia, including three individuals affected by both spasticity and dystonia. Thirteen individuals (39.4%) presented with a change in communication skills, including loss of vocabulary (n=8), decreased speech production (n=9), or dysarthria (n=5). Cranial nerve deficits (n=2) and ischemic strokes (n=2, both associated with Moya Moya vasculopathy) were also observed. Ten individuals (30.3%) presented with systemic features, including sterile pyrexia (n=4), feeding intolerance (n=1), and myalgias (n=2). Three patterns of clinical decline during the fulminant period were identified: acute (nadir within 1 week, n=5), subacute (1–4 weeks to reach nadir, n=11), and chronic (nadir reached over 4 weeks after fulminant onset, n=13) (Figure 2E). The remainder of patterns of decline were unknown.
To evaluate the time to diagnosis in later-onset, atypical AGS, we collected information on the time from disease onset to medical evaluation. The median time from fulminant symptom onset to first medical encounter was 4 days (range 0 – 197 days). The median time from fulminant symptom onset to first MRI was 33 days (range 0 days - 2 years) and the time to AGS diagnosis was 1.25 years (median, range 0 – 20 years).
Next, we evaluated the process of diagnosis in atypical AGS cases. The most common (non-mutually exclusive) diagnoses included congenital spastic diplegia or cerebral palsy (n=4), acute demyelinating encephalomyelitis (ADEM) (n=4), and post-viral encephalitis (n=4). Other considerations included non-specified leukodystrophy (n=9), metabolic/mitochondrial disease (n=6), non-specified genetic syndrome (n=2), and behavioral disturbance (n=1). Two individuals had initial symptoms that were attributed to orthopedic injuries (transverse tibial fracture and hyperextension deformity of the knee).
The results from early cerebral imaging was available for review in the majority of cases (n=31), with images available for 13 cases (Figure 3). These images were obtained a median of 0.1 years after the onset of fulminant symptoms (range 0.0–2.0 years). White matter abnormalities (n=16, 51.6%) were the most commonly noted radiographic feature within our atypical AGS cohort. This included delayed myelination (ADAR1, n=2; IFIH1, n=3; RNASEH2B, n=3), large multifocal lesions (n=8), and small focal abnormalities (Figure 3E, n=5). Of note, the imaging of one individual demonstrated multiple large lesions as well as a small focal parietal lesion. The localization of white matter injury was variable, but was predominantly supratentorial involving the periventricular region and deep matter. One individual demonstrated near-confluence of the white matter changes (Figure 3D).
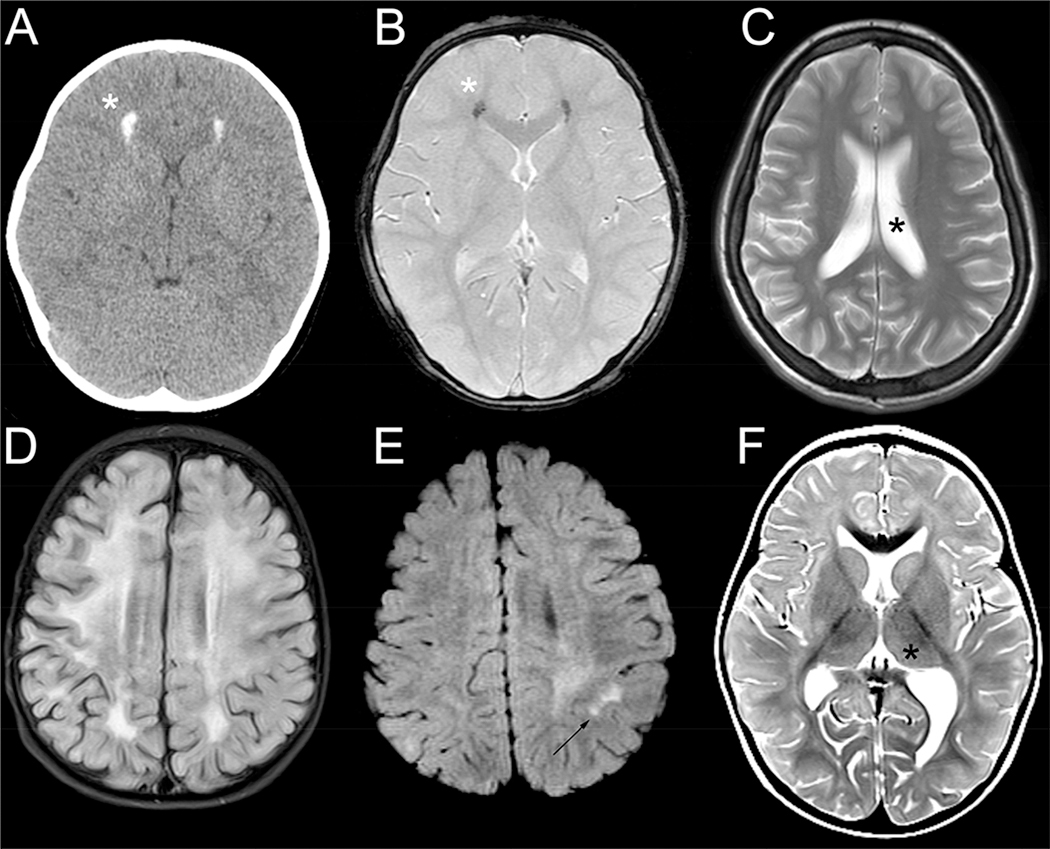
Figure 3.
Imaging features found in atypical AGS. Imaging findings include calcifications (*) as visualized by head CT (A) and GRE (B) as demonstrated in the same individual, mild ventriculomegaly on T2 imaging (*, C), diffuse white matter changes as demonstrated by T2 FLAIR hyperintensity (D), patchy white matter hyperintensities (T2 FLAIR, arrow, E), and basal ganglia and thalami involvement on T2 (*).
The common features of neonatal AGS were largely absent on these initial images. Only 4 individuals (12.9%) had evidence of calcifications (Figure 3A–B), and no individuals were noted to have cystic lesions. As assessed by T2 hyperintensity, involvement of the thalamus (ADAR1, n=1; Figure 3F) and basal ganglia (ADAR1, n=7; RNASH2B, n=1, SAMHD1, n=2) was noted in a subset of individuals. Nine individuals (29.0%) were noted to have mild ventriculomegaly (Figure 3C). Three individuals with SAMHD1-related disease were noted to have vascular involvement: two with Moya Moya syndrome with infarcts and another individual with MCA-ICA stenosis with possible calcification of the vessels. The most common radiographic diagnoses from the original reports were leukodystrophy (n=14 of 25 available reports, 56.0%), metabolic disorders (n=7, 28.0%), and ADEM or encephalitis not otherwise specified (n=2 each, 8.0%).
Four individuals with presumed neuroinflammatory disease received immunomodulatory therapy within one month of their fulminant presentation: intravenous (IV) steroids followed by enteral steroids (n=2), IV steroids with intravenous immunoglobulin (IVIG) (n=1), and oral steroids alone (n=1). While all 4 individuals showed partial improvement, none of these individuals had a resolution of symptoms with these immunomodulatory approaches.
Discussion
Aicardi Goutières Syndrome is a genetic autoinflammatory leukoencephalopathy with significant phenotypic variability [1, 4, 25, 31]. The goal of our study was to characterize the atypical, later onset cases within our international cohort. Our results suggest that the signs and symptoms AGS often predate fulminant neurologic decline. This has important implications related to the timing of future clinical interventions and underscores the need for early screening platforms, such as newborn screening [2]. The first ‘early’ phase was characterized by chronic symptoms including global developmental delay or subtle symptoms of systemic inflammation; the prodromal phase was characterized by worsening systemic inflammatory symptoms.
The fulminant neurologic regression was characterized by the loss of motor skills often accompanied by a change in language function and a history of subtle systemic symptoms, including sterile pyrexias, myalgias, and GI intolerance. These features were often different from the classic systemic features noted in the neonatal and infantile forms of the disease, which include altered mental status, irritability, seizures, failure to thrive, hepatitis, or blood dyscrasia [32]. In contrast to prior studies characterizing AGS as an acute or subacute process [4, 32], half of our cohort developed worsening neurologic function over a period of time longer than a month.
One of the most common misdiagnoses in the atypical AGS population was ADEM. Of note, none of the individuals within our cohort diagnosed with ADEM had altered mental status (including irritability or confusion) at the time of fulminant presentation, which is part of the diagnostic criteria for ADEM. Another common misdiagnosis was congenital spastic diplegia, despite the fact that disease onset developed after one year of life.
The imaging findings within this atypical AGS cohort were diverse, but most demonstrated evidence of white matter involvement. Classic early-onset AGS findings of calcifications and cerebral atrophy were noted in minority of MR images. We hypothesize that this may be due to the proximity of the imaging to the onset of fulminant symptoms. Only one of our atypical cases was noted to have bilateral striatal necrosis at disease onset, as has been previously described with ADAR1-related AGS [9]. This underscores the need to more broadly consider AGS regardless of the MRI features noted at onset. This study focused on the retrospective information up to the time of fulminant disease onset, and serial, subsequent imaging was not reviewed. Future prospective studies will be better suited to the characterization of the evolution of imaging findings after disease onset.
The individuals in this cohort treated with immunomodulatory therapies demonstrated an incomplete and transient improvement. These immunomodulatory therapies did not result in a sustained improvement in neurologic function or a change in the neurologic trajectory associated with AGS. In the first months after fulminant disease onset, individuals with AGS can demonstrate fluctuating neurologic function [25]. We hypothesize that the improvement attributed to the immunomodulatory therapies may have been due to natural fluctuations in the disease.
Our study has several limitations. The inclusion criteria may have excluded atypical cases presenting at less than one year of age. As our cohort is also limited by ascertainment bias, we hypothesize that even further diversity in the AGS-spectrum exist as part of an AGS genes-related type I interferonopathy spectrum. Our study is also limited by the available clinical information from provider notes. For example, typically only MR images were obtained in the early phase of disease, while CT images, which are more sensitive to calcifications, were not available in the majority of subjects. Additionally, early diagnostic testing results, including cerebrospinal fluid cell counts and markers of inflammation were not consistently available across the cohort. Despite these limitations, we were able to demonstrate that later, atypical AGS cases have a pattern of evolution: from early subtle symptoms, to worsening systemic inflammation, and culminate in neurologic decline. This encompasses subtypes previously classified as spastic paraparesis. As such we would recommend consideration of AGS in the differential diagnosis for young children presenting clusters of AGS-associated symptoms of systemic inflammation, particularly when accompanied by a change in neurologic function.
This is the first study to characterize late-onset AGS and underscores the broad phenotypic and radiographic spectrum found in this disorder. Early recognition is critical, particularly as new, targeted therapeutic options are developed. We encourage practitioners to consider AGS in their broader list of potential diagnoses and within their comprehensive diagnostic evaluation.
Acknowledgments
Funding support:
AV: Supported by the Kamens endowed chair for Translational Neurotherapeutics and the Myelin Disorders Bioregistry Project, as well as NIH-funded grants (U54TR002823, 1U01NS106845). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
LA: Research reported in this publication was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number K23NS114113 and U54TR002823. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SO, VDG, CV: Research reported in this publication was supported by the Italian Health Ministry grant RC2017-19 to IRCCS Mondino Foundation, Pavia
Conflicts:
AV: Receives research support from Gilead Sciences Inc., Homology Medicines, Eli Lilly and Company, Shire/Takeda, Ionis, Biogen and Ilumina Inc. She serves on the scientific advisory board of the MLD Foundation, the United Leukodystrophy Foundation, and the European Leukodystrophy Foundation. She is a consultant to Orchard Pharmaceutical. She has provided unpaid scientific advisory services to Illumina, Shire/Takeda, Ionis and Biogen, in addition to the research support she receives.
LA: Serves on the MLD Foundation Medical/Scientific Advisory Board and the CureMLD Scientific Advisory Board. She is a consultant to Orchard Pharmaceutical.
DT: Serves on the ULF Medical/Scientific Advisory Board Provided scientific advisory services to Ionis.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.: Seattle (WA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adang L, et al. , Developmental outcomes of Aicardi Goutières Syndrome. Journal of Child Neurology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armangue T, et al. , Neonatal detection of Aicardi Goutieres Syndrome by increased C26:0 lysophosphatidylcholine and interferon signature on newborn screening blood spots. Mol Genet Metab, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston JH and Crow YJ, Neurologic Phenotypes Associated with Mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi-Goutieres Syndrome and Beyond. Neuropediatrics, 2016. 47(6): p. 355–360. [DOI] [PubMed] [Google Scholar]
- 4.Crow YJ, et al. , Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A, 2015. 167a(2): p. 296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aicardi J and Goutieres F, A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol, 1984. 15(1): p. 49–54. [DOI] [PubMed] [Google Scholar]
- 6.Gilani A, et al. , Neuropathological Findings in a Case of IFIH1-Related Aicardi-Goutieres Syndrome. Pediatr Dev Pathol, 2019: p. 1093526619837797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin B, et al. , Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proc Natl Acad Sci U S A, 2011. 108(13): p. 5372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svingen L, et al. , Late diagnosis and atypical brain imaging of Aicardi-Goutieres syndrome: are we failing to diagnose Aicardi-Goutieres syndrome-2? Dev Med Child Neurol, 2017. 59(12): p. 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston JH, et al. , A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J Med Genet, 2014. 51(2): p. 76–82. [DOI] [PubMed] [Google Scholar]
- 10.Rice GI, et al. , Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol, 2013. 12(12): p. 1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim YW, et al. , Genome-wide DNA hypomethylation and RNA:DNA hybrid accumulation in Aicardi-Goutieres syndrome. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalves A, et al. , SAMHD1 is a nucleic-acid binding protein that is mislocalized due to aicardi-goutieres syndrome-associated mutations. Hum Mutat, 2012. 33(7): p. 1116–22. [DOI] [PubMed] [Google Scholar]
- 13.Orebaugh CD, et al. , The TREX1 exonuclease R114H mutation in Aicardi-Goutieres syndrome and lupus reveals dimeric structure requirements for DNA degradation activity. J Biol Chem, 2011. 286(46): p. 40246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YG, Lindahl T, and Barnes DE, Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell, 2007. 131(5): p. 873–86. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Hwang SY, and Ahn K, Interplay between RNASEH2 and MOV10 controls LINE-1 retrotransposition. Nucleic Acids Res, 2018. 46(4): p. 1912–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benitez-Guijarro M, et al. , RNase H2, mutated in Aicardi-Goutieres syndrome, promotes LINE-1 retrotransposition. EMBO J, 2018. 37(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann A, et al. , The SAMHD1-mediated block of LINE-1 retroelements is regulated by phosphorylation. Mob DNA, 2018. 9: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao K, et al. , Modulation of LINE-1 and Alu/SVA retrotransposition by Aicardi-Goutieres syndrome-related SAMHD1. Cell Rep, 2013. 4(6): p. 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao K, et al. , LINE1 contributes to autoimmunity through both RIG-I- and MDA5-mediated RNA sensing pathways. J Autoimmun, 2018. 90: p. 105–115. [DOI] [PubMed] [Google Scholar]
- 20.Chung H, et al. , Human ADAR1 Prevents Endogenous RNA from Triggering Translational Shutdown. Cell, 2018. 172(4): p. 811–824.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orecchini E, et al. , ADAR1 restricts LINE-1 retrotransposition. Nucleic Acids Res, 2017. 45(1): p. 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice GI, et al. , Genetic, Phenotypic, and Interferon Biomarker Status in ADAR1-Related Neurological Disease. Neuropediatrics, 2017. 48(3): p. 166–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice GI, et al. , Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet, 2014. 46(5): p. 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice G, et al. , Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet, 2007. 81(4): p. 713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adang LA, et al. , Development of a neurologic severity scale for Aicardi Goutieres Syndrome. Mol Genet Metab, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Piana R, et al. , Neuroradiologic patterns and novel imaging findings in Aicardi-Goutieres syndrome. Neurology, 2016. 86(1): p. 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klok MD, et al. , Interferon-alpha and the calcifying microangiopathy in Aicardi-Goutieres syndrome. Ann Clin Transl Neurol, 2015. 2(7): p. 774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Salam GMH, et al. , Aicardi-Goutieres syndrome: unusual neuro-radiological manifestations. Metab Brain Dis, 2017. 32(3): p. 679–683. [DOI] [PubMed] [Google Scholar]
- 29.Tonduti D, et al. , Spontaneous MRI improvement and absence of cerebral calcification in Aicardi-Goutieres syndrome: Diagnostic and disease-monitoring implications. Mol Genet Metab, 2019. 126(4): p. 489–494. [DOI] [PubMed] [Google Scholar]
- 30.Crow YJ, et al. , Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics, 2014. 45(6): p. 386–93. [DOI] [PubMed] [Google Scholar]
- 31.Rice GI, et al. , Genetic and phenotypic spectrum associated with IFIH1 gain-of-function. Hum Mutat, 2020. 41(4): p. 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crow YJ, Aicardi-Goutieres Syndrome, in GeneReviews(R), Adam MP, et al. , Editors. 1993, University of Washington, Seattle [Google Scholar]