Fig. 4.
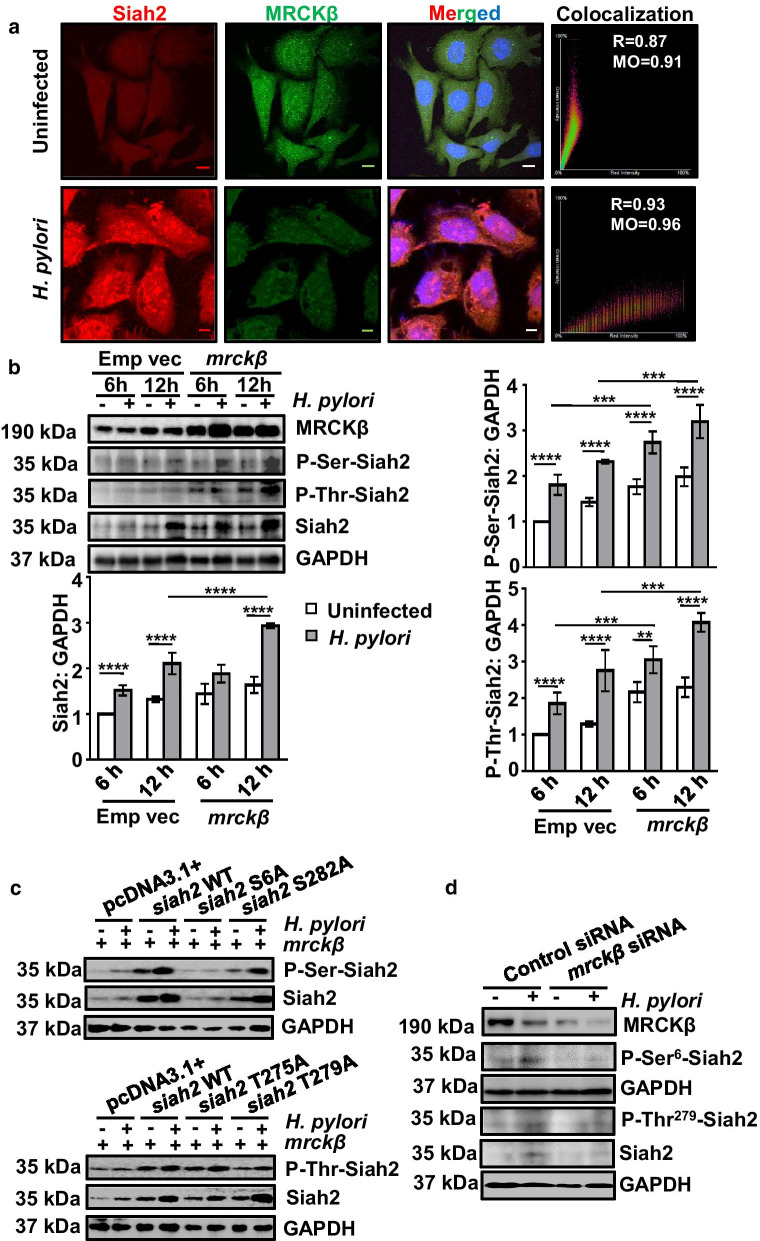
MRCKβ potentiates Siah2 phosphorylation in GECs after H. pylori infection. a Confocal images (n = 3) of uninfected and H. pylori-challenged (200 MOI for 12 h) AGS cells representing subcellular localization of Siah2 and MRCKβ proteins. Individual DAPI-stained panels showing nuclear staining are not shown due to constraint of space but the merged panels include DAPI. Scale bar represents 5 μm. Scatter plots generated by using NIS AR software represent co-localization of MRCKβ and Siah2 along with their Pearson's correlation (R) and Mander’s overlap (MO) values. b A representative (n = 3) western blot of whole cell lysates of MKN45 cells transfected with pcDNA3.1+ and mrckβ and infected with H. pylori showing MRCKβ, P-Ser-Siah2, P-Thr-Siah2 and Siah2 protein status. GAPDH = loading control. Bar graphs indicate increase of P-Ser-Siah2, P-Thr-Siah2 and Siah2 after mrckβ overexpression followed by 12 h of H. pylori infection. Two-way ANOVA followed by Tukey’s post hoc analysis are performed to evaluate statistical significance. Data are mean ± sem (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001. c Representative western blot (n = 3) of pcDNA3.1+, siah2 WT and Siah2 phospho-null mutant-expressing MKN45 stable cells transfected with mrckβ followed by H. pylori infection showing P-Ser-Siah2 and Siah2 (above) and P-Thr-Siah2 and Siah2 (below). GAPDH is the loading control. d Representative western blot (n = 3) from whole cell lysate of MKN45 cells transfected with either control or mrckβ siRNA followed by H. pylori infection indicating MRCKβ, P-Ser6-Siah2, P-Thr279-Siah2 and Siah2 proteins. GAPDH is kept as a loading control