Figure 1.
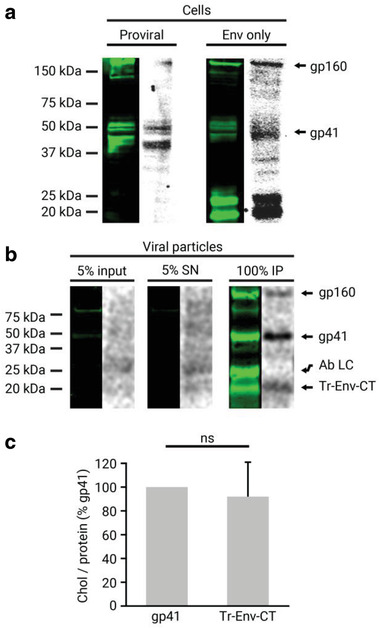
Gp41 interaction with chol in cells and released mature viral particles. a) Gp41 was immunoprecipitated from HEK 293T cells fed with the chol analogue [3H]‐photochol and detected by Western blot with the anti‐gp41 chessie‐8 antibody (green lanes). The interacting radioactively labeled lipid was detected by autoradiography of the same membrane (gray lanes). Cells were transfected either with a proviral pCHIV plasmid expressing all of the viral proteins or with an Env only expressing pCAGGS plasmid. b) Viral particles were purified from HEK 293T cells transfected with the pCHIV proviral plasmid and treated with the chol analogue [3H]‐photochol. Gp41 from input (5% of lysed viral particles), supernatant (SN; 5% of total), and immunoprecipitation (IP) from purified viral particles were detected by Western blot with the anti‐gp41 chessie‐8 antibody (green lane), and the interacting radioactively labeled lipid by autoradiography of the same membrane (gray lane). Protein bands corresponding to gp160, gp41, the light chain of the antibody used for immunoprecipitation (Ab LC), and a truncated form of gp41 (Tr‐Env‐CT) are labeled. c) Quantification of the chol/protein signal ratio of the Tr‐Env‐CT protein fragment compared to the full‐length gp41. The bars and whiskers represent the mean ± SD of experiments from five independent viral purifications (n = 5). Statistical significance was assessed by analysis of variance and Tukey test.
