Figure 3.
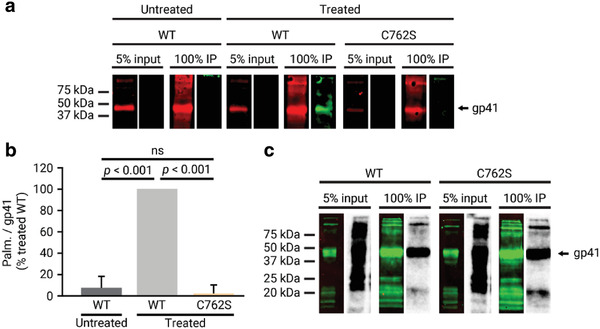
Effect of the C762S substitution on gp41 palmitoylation and gp41–chol interaction in purified mature viral particles. a) Gp41 from input (5% of lysed viral particles) and immunoprecipitation (IP) from viral particles treated or not with the clickable palmitoyl analogue precursor 15‐hexadecynoic acid were detected by Western blot with the anti‐gp41 chessie‐8 antibody (red), and the palmitoyl group of the protein was detected by the covalent binding of a IRDye800 azide fluorescent probe mediated by a click‐chemistry reaction (green). b) Quantification of the palmitoyl/protein signal ratio of the mutated gp41 protein C762S compared to the wild‐type gp41 from either treated or untreated viral particles. The bars and whiskers represent the mean ± SD of experiments from three independent viral purifications (n = 3). Statistical significance was assessed by analysis of variance and Tukey test. c) Gp41 from input (5% of lysed viral particles) and immunoprecipitation (IP) from viral particles were detected by Western blot with the anti‐gp41 chessie‐8 antibody (green lane), and the interacting radioactively labeled lipid by autoradiography of the same membrane (gray lane).
