Figure 5.
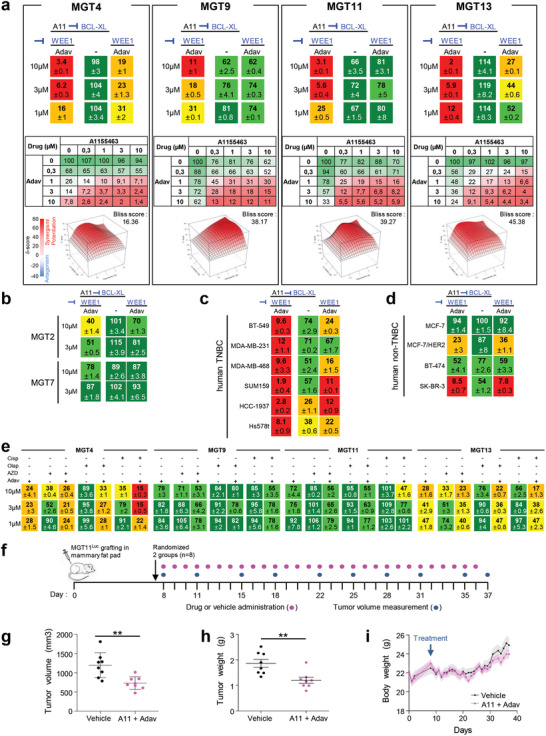
Combined inhibition of BCL‐XL and WEE1 is deleterious for all MMTV‐R26Met MGT and human TNBC cell lines tested. a) Dose–response effects of A1155463 (A11, targeting BCL‐XL) alone or in combination with Adavosertib (Adav, targeting WEE1) on the viability of the four tumorigenic MMTV‐R26Met MGT cell lines. Combined drug effects are reported on the left of the top panel. Detailed matrix (middle panel) and Loewe plots (lower panel) highlight the drug synergism. b) Cell viability assay performed on the nontumorigenic MGT2 and MGT7 cell lines highlights the lack of in vitro toxic effect of A1155463+Adavosertib drug combination. c,d) Cell viability in response of A1155463 (1 × 10−6 m) and Adavosertib (3 × 10−6 m) in a panel of c) human TNBC and d) human non‐TNBC breast cancer cell lines. In all figures, cell viability is presented as percentage of control (untreated cells) and labeled by the green (high)‐to‐red (low) color code. e) Dose–response effects on the viability of MMTV‐R26Met MGT cells treated with single or combined drugs as indicated. In (a–e), at least three independent experiments were performed. Values are expressed as the mean ± s.e.m. f–i) In vivo effects of A1155463+Adavosertib treatment in orthotopic tumors. (f) Orthotopic injection of MGT11Luc cells in the mammary fat pad of NSG mice, drug administration, and tumor volume measurement were performed as illustrated. Tumor volume (g) and tumor weight (h) measured at the end point of the experiment (day 37; n = 8 mice per group). (i) Graph reporting the evolution of the body weight of mice during the whole procedure. Body weight was measured every day, before drug administration. No significant changes were observed, indicating that the dose of drugs used in vivo were not toxic. A11: A1155463 (BCL‐XL inhibitor); Adav: Adavosertib (WEE1 inhibitor); AZD: AZD6738 (ATR inhibitor); Cisp: cisplatin; Olap: Olaparib (PARP inhibitor). Values are expressed as the mean ± s.e.m. Unpaired Student's t‐test was used. ** P < 0.01.
