Abstract
Purpose:
The aim of this study was to discuss the possible risk factors predisposing to post photorefractive keratectomy (PRK) haze formation and develop and validate a risk scoring system, so that this could be applied to our clinical practice as an algorithmic approach.
Methods:
Study was divided into 2 arms, in the retrospective arm we looked at 238 eyes of patients undergoing PRK where certain presumed risk factors from literature and clinical experience were identified and statistical significance of association was studied in the development of corneal haze. The risk scoring system was applied to the 450 eyes in the prospective arm for validation. This was then used to formulate an algorithmic approach to manage post-PRK haze.
Results:
22 out of 238 eyes in the retrospective arm developed haze where risk factors such as contact lens intolerance, altered tear film break up time, meibomian gland drop out and vitamin d levels were significantly associated with post-PRK haze (p < 0.05) and these factors were identified in the prospective arm. Treatment of these modifiable factors led to a significant reduction in post-PRK haze.
Conclusion:
Thus identifying and treating risk factors of haze in patients undergoing PRK could improve surgical outcomes and patient satisfaction.
Keywords: Haze, management, prediction, PRK, risk
Photorefractive keratectomy (PRK) is an FDA (Food and Drug Administration, USA) approved procedure for laser vision correction in which the excimer laser is applied after corneal epithelial removal.[1] It has been shown to cause less biomechanical weakening than other corneal refractive procedures, and is therefore the procedure of choice in patients who have thinner corneas and subtle topographic irregularities unsuitable for Laser in Situ keratomileusis (LASIK).[2] However, this procedure can also have side effects early or late-onset corneal haze which may cause a significant reduction in the postoperative vision.[3,4,5] It is therefore important to find methods to minimize post-PRK haze to optimize outcomes.[6,7] A number of factors have been implicated in post-PRK haze including tissue ablation for high refractive errors, laser energy used, size of ablation zones, methods of epithelium removal, amount of postoperative UV exposure,[8,9] and even autoimmune conditions.[10] A subset of patients develops significant post-PRK haze even without these known risk factors.[11,12] Mitomycin-C (MMC) is an antimetabolite which is used intraoperatively to reduce the incidence of post PRK haze. It acts by modulating wound healing by inhibiting myofibroblast formation and keratocyte activation implicated in formation of subepithelial haze.[11,13,14] However, in spite of its use, patients can still develop visually significant haze.[15]
Investigating lesser-known potential contributing factors based on existing literature like ocular surface inflammation in contact lens usage, dry eye disease,[16] nutritional factors like vitamin D levels,[17] age and gender[18] could open newer avenues to prevent or treat post PRK haze.
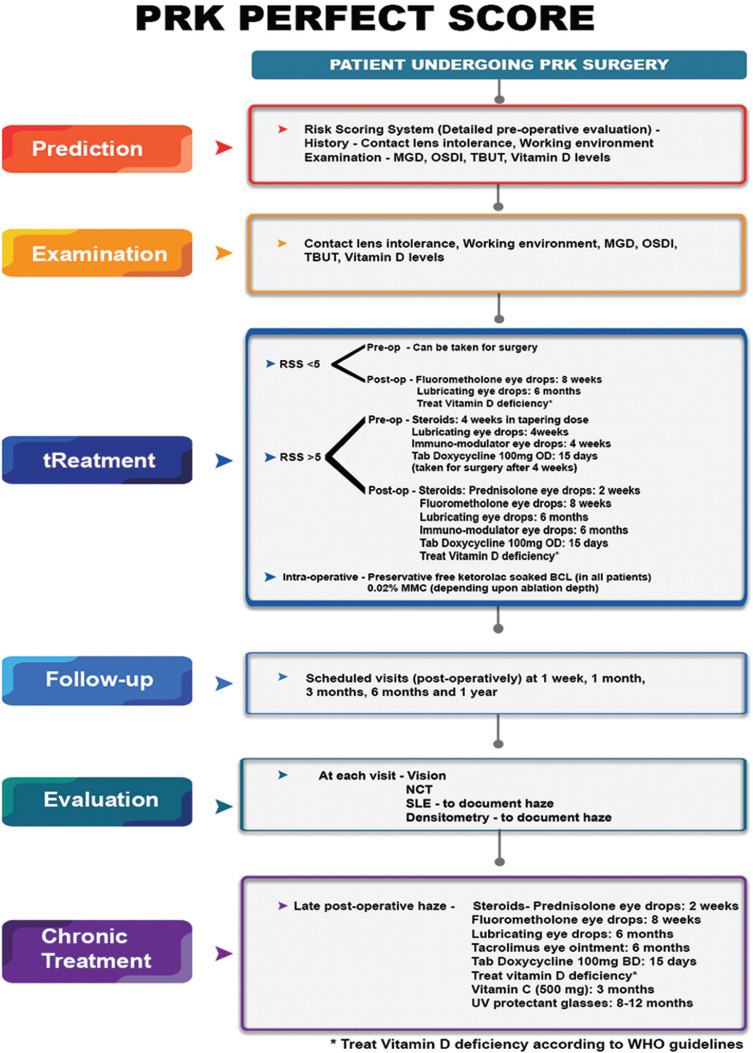
The aim of this study was to discuss the identified risk factors predisposing to post PRK haze and develop and validate a risk scoring system to be applied to clinical practice. Based on this an algorithmic evaluation and management protocol has been formulated for the clinic. We have named this the PRK PERFECT Protocol, which stands for- Prediction, Examination, tReatment, Follow-up and Chronic Treatment).
Methods
The study has a retrospective arm and a prospective arm. The collection and analysis of all the study data was done according to the Declaration of Helsinki with Institutional ethics committee approval. An informed, written consent was obtained from all the patients. The inclusion and exclusion criteria, preoperative evaluation, and postoperative care were same for both the retrospective and prospective arms and all the patients were operated by the same surgeon.
Patients between the age of 20 to 40 years with refractive error between − 2.00 D to − 6.00 D of myopia and astigmatism less than 3D were included in the study. Other criteria for surgical selection were as per standard clinical practice and patients with abnormal corneal topography unsuitable for refractive surgery, history of previous ocular surgery, ocular infection, any corneal ectatic condition, and connective tissue disorder were excluded from surgery.
Preoperative assessment
A detailed history for refractive stability, contact lens usage and intolerance, dry eye, allergies, pregnancy, systemic illness including keloid tendency, type of work and working environment was taken. Preoperative workup included uncorrected (UDVA) and corrected (CDVA) distant visual acuity, manifest refraction, cycloplegic refraction, dry eye tests including Schirmer's, tear break up time, meibography and ocular surface staining and corneal tomography using Pentacam HR (OCULUS Optikgerate GmbH, Wetzlar, Germany).
Post-operative care
Standard postoperative regimen and evaluation was followed for all patients in both the arms. Topical moxifloxacin hydrochloride 0.5% eye drops for one week and Fluorometholone 0.1% eyedrops at 4 times per day and tapered for 12 weeks. The postoperative follow-up visits were scheduled at 1 day, 3 days, one week, one month, 3 months, 6 months and 1 year. BCL was removed on the 3rd day after surgery. Visual acuity (UDVA, CDVA) was measured using Snellen acuity chart. Intra-ocular pressure was documented by non-contact tonometer after bandage contact lens removal. All subjects were screened during follow-up for the development of vision compromising corneal haze. Stromal haze was assessed clinically on slit-lamp examination based on the grading system by Fantes et al.[19] and a 2-fold increase in gray-scale units (GY) on Scheimpflug densitometry of Pentacam.[20]
Retrospective arm
All patients who underwent PRK between 2013 and 2016 and fulfilling our inclusion/exclusion criteria were included in the retrospective arm.
Surgical procedure
Details of surgery were obtained from the patient case records. Manual epithelium removal by mechanical debridement of 6 mm diameter was done. Excimer laser ablation was performed using Wavelight EX500 (WaveLight® EX500 Excimer Laser; Alcon Laboratories, Ft Worth, TX, USA) and 0.02% MMC (0.2 mg/ml) was applied on the stromal bed after ablation, at 10 seconds per dioptre correction in patients with more than 2 D refractive error.[21] The MMC was thoroughly washed with balanced salt solution and a bandage contact lens (BCL; Ciba Vision, Duluth, GA) was placed.
This group of patients were divided into those who developed clinically significant haze as defined previously (Group 1) and those who did not (Group 2 control). Certain presumed risk factors from literature and clinical experience were identified in this group like dry eye disease (DED)[22] and contact lens intolerance,[23] high Ocular surface disease index (OSDI) scoring[24] and increased meibomian gland dropouts.[25] Systemic factors like vitamin D have been known to play a role in corneal wound healing and could have therapeutic implications.[26,27] Since UV exposure is an identified risk factor for haze development, outdoor work was defined as activity of >6 hours[8,28] These risk factors were then analyzed individually against the presence or absence of corneal haze and a relative risk was assessed. Hypothesizing that patients having more than one risk factor could be at a higher risk of developing haze, we devised a risk scoring system [Fig. 1].
Figure 1.
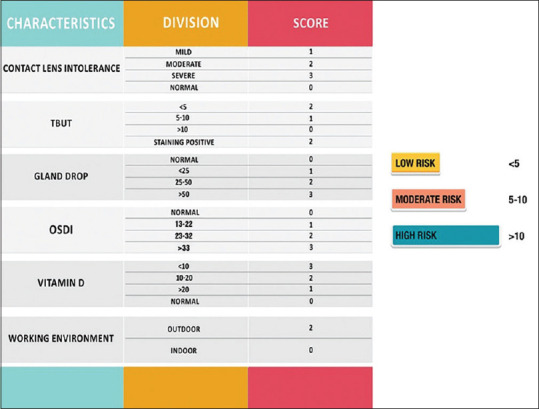
Risk Scoring system for post PRK haze
Prospective arm
To validate this risk scoring system, we applied it to a prospective group of 450 eyes of 225 patients planned for PRK and treated the modifiable risk factors before surgery. We term this the PERFECT PRK protocol, which stands for- Prediction, Examination, tReatment, Follow-up and Chronic Treatment).
The surgical procedure performed in this group was single-step trans-PRK using Schwind Amaris excimer laser1050RS (SCHWIND eye-tech-solutions) keeping an optical zone of 6 mm. MMC 0.02% was used as per the same protocol followed in the retrospective arm. A bandage contact lens (BCL; Ciba Vision, Duluth, GA) soaked in preservative-free ketorolac tromethamine 0.45% (Acuvail® ophthalmic solution) was placed over the cornea after the PRK procedure to alleviate pain.[29] The same postoperative management, follow up regimen and evaluation was followed as in the retrospective group.
Statistical analysis
The analysis of the data was done using MedCalc Version 19.4.1. All parameters were assessed for normality of distribution. Continuous variables were tested using difference in their mean/median. Differences in proportion of individual risk factors for post-operative haze between cases and controls were assessed using appropriate tests of proportion.
Results
The retrospective arm of the study comprised of 238 eyes of 119 patients who underwent PRK between 2013 and 2016. The demographic, refractive and keratometric parameters of 119 patients who underwent PRK are listed in Table 1. 22 out of 238 eyes developed clinically significant postoperative haze- group 1 which lasted for 6 months. Out of which 2 eyes developed late-onset corneal haze after 3 months of surgery. Age and gender-matched subjects who underwent the same procedure, but did not develop haze were considered as controls (Group 2) [Fig. 2a-c]. The risk factors were compared between the two groups to study their association with post PRK haze and relative risks have been enumerated in [Tables 2 and 3]. There was a strong association and significantly higher relative risk of developing PRK haze with contact lens intolerance, dry eye, high OSDI, vitamin D deficiency and outdoor work (p < 0.05). No significant association was found between development of haze and age or gender of the patient. A risk assessment score was formulated based on the number of risk factors in each patient [Fig. 1].
Table 1.
The table provides mean±sem values of the corneal thickness
| HAZE patient cohort | |||
|---|---|---|---|
| Group without haze controls (n=96 eyes) | Group with haze (n=22 eyes) | P | |
| Age | 24.73±1.96 | 24.33±1.33 | 0.85 |
| K1 (D) | 43.14±0.21 | 43.23±1.89 | 0.91 |
| K2 (D) | 45.24±0.32 | 45.67±2.01 | 0.65 |
| Km (D) | 44.03±0.32 | 44.43±1.96 | 0.71 |
| K-Max (D) | 45.69±0.48 | 46.43±1.33 | 0.88 |
| MRSE (D) | −3.15±0.42 | −3.9±0.17 | 0.09 |
K1, K2: Independent readings of corneal curvature by keratometry; Km and K-Max: Mean keratometry value and maximum keratometry value respectively. MRSE: Manifest refraction spherical equivalent, The ANOVA P value column shows group statistics
Figure 2.
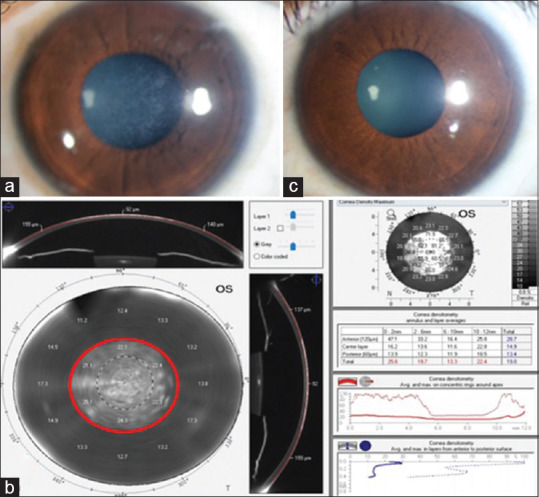
Clinical images illustrating corneas which underwent PRK surgery using Slit lamp bio microscopy (a) Corneal haze subject: cornea of grade 2 subepithelial corneal haze 12 months post PRK. (b) To visualize post PRK haze, the densitometry mapping by Oculus Pentacam shows absolute values in different zones within normal range for control with increase in Gray scale units (GY) at 0–2, 2–6- and 6–10-mm zone in the anterior 120 micrometer in corneal haze subject is shown (c) Control subject: clear cornea 12 months post PRK.
Table 2.
Association of each risk factor to patients developing haze post PRK
| Group 1 with haze (n=22 eyes) | Group 2 without haze controls (n=96 eyes) | P | |
|---|---|---|---|
| CL intolerance | 10 | 6 | 0.03 |
| TBUT <10 sec | 12 | 23 | 0.17 |
| OSDI> 33 | 14 | 4 | 0.02 |
| Meibomian gland drop out (>50%) | 15 | 6 | 0.04 |
| Vitamin D levels (<10 ng/ml) | 14 | 12 | 0.04 |
| Working environment (outdoor) | 12 | 34 | 0.09 |
Table 3.
Odds ratio (OR) and relative risk (RR) for each of the risk factors in the patients which developed haze versus those which did not develop haze post PRK
| OR | 95% CI For OR | P for OR | RR | 95% CI For RR | P for RR | |
|---|---|---|---|---|---|---|
| Contact lens intolerance | 11.3 | 3.3-38 | 0.001 | 4.785 | 2.4-9.3 | <0.001 |
| TBUT <10 sec | 3.8 | 1.45-9.95 | 0.006 | 2.27 | 1.35-3.83 | 0.002 |
| OSDI >33 | 30.2 | 10.6-151 | <0.0001 | 15.27 | 5.5-41.9 | <0.0001 |
| Meibomian gland drop out (>50%) | 32.14 | 9.49-108.84 | <0.0001 | 10.9 | 4.77-24.91 | <0.001 |
| Vitamin D levels (<10 ng/ml) | 12.25 | 4.25-35.3 | <0.0001 | 5.09 | 2.7-9.4 | <0.0001 |
| Working environment (outdoor) | 5.47 | 1.52-19.6 | 0.009 | 2.11 | 1.3-3.4 | 0.02 |
Prospective arm
Prospectively, we applied this scoring to 450 eyes of 225 patients planned for PRK.
Out of 450 eyes 88 eyes were categorized as high risk, 128 eyes as moderate risk and 234 eyes as low risk of developing haze. Risk factors which could be modified, such as pre-operative ocular surface inflammation due to contact lens intolerance, dry eye or abnormal TBUT and low vitamin D levels were adequately treated preoperatively [Fig. 3]. The overall incidence of postoperative haze in the prospective PRK group after treating the identified risk factors was 2% which was lower than the retrospective arm (9%). 4 out of 88 eyes (4%) in the high risk group, 3 out of 128 eyes (2.5%) in the moderate risk group and 1 out of 234 eyes (0.4%) in the low risk group developed post PRK haze.
Figure 3.
Proposed algorithm for management of post PRK haze
Discussion
Proper wound-healing after surgery is an important determinant of good outcomes for all surgeries and especially in PRK. After surgery, there is a release of inflammatory markers like TNF-α, MMP-9, interleukin (IL)-1α and IL-1β into the stroma leading to the activation of keratocytes and their differentiation into myofibroblasts.[30] Situations in which there is increased inflammation, irregular stromal surface and increased keratocyte death as seen after ablation for high refractive errors can result in defective EBM regeneration and more inflammation in the stroma.[16] This results in deposition of disorganized cellular material, abnormal extracellular stromal remodeling and haze formation.[31,32] Thus treating ocular surface inflammation preoperatively is important. Additional factors like nutritional deficiency, environment inflammation, UV-B rays, atopy, autoimmune diseases, keloid and age may lead to the development of late-onset haze.[8,18] Many patients opt for refractive surgery due to CL intolerance.[33] In our study, we found a significant association of preoperative CL intolerance to post PRK haze. Thus, PRK in a CL intolerant eye without treating the inflamed ocular surface can have an altered healing and contribute to increased haze. Meibomian gland dropout, low preoperative TBUT and higher OSDI were also found to be associated with increased post-operative haze in our case series (P < 0.05). This is attributed to the instability of tear film which leads to stress in the ocular surface cells and inflammatory cascade.[25,34,35] The OSDI questionnaire score has been shown to be a good marker of ocular surface inflammation.[36] This could explain the increased haze in this sub-group of our cohort. The role of systemic vitamin D deficiency could be explained by the fact that Vitamin D plays a role in modulating corneal wound healing.[37,38] Studies have demonstrated both age and gender to be significant risk factors for development of corneal haze[18] but we did not find a significant association in our cohort. (p = 0.07) The role UV light was corroborated in this study as well.[8] Postoperative medications and care are integral to the entire treatment ritual and help in achieving good surgical outcomes. Topical corticosteroids post-PRK inhibit the activation of fibrocytes and thus, play an important role in controlling corneal haze. Longer use of topical steroids with monitoring for complications like rise in intraocular pressure is advisable.[39] Preoperative management inflammation is essential to improve postoperative outcomes. Topical immuno-modulators like cyclosporine 0.05% eye drops for 6 months have a lower risk profile, steroid-sparing effect and help in controlling chronic inflammation post-PRK[39,40] Nutritional supplements with Vitamin D[37] and vitamin C may be beneficial.[40]
Limitations-One of the limitations of our study is that we have studied patients undergoing PRK only up to -6D myopia. Higher ablations are not studied. Another is that a larger sample size of patients may give more information as the incidence of haze is in itself low. An important point is the difference in surgical technique between the 2 groups (manual epithelial removal in the retrospective arm and transepithelial laser ablation in the prospective arm) which could also have a bearing on the results and therefore a confounder.[41,42] The different excimer lasers used in the 2 groups could also have a bearing on the haze formation.[43]
Conclusion
Postoperative haze is one the most important complications of the PRK procedure. There are a number of risk factors that have been associated with the development of PRK haze. An in-depth understanding of the entire wound healing process could help us identify lesser-known risk factors. The PRK PERFECT protocol [Fig. 3] allows adequate categorization of the patients according to the risk scoring system. Treatment measures at each step of the surgery can lead to improved outcomes post PRK and decrease the incidence of post PRK haze.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Seiler T, Wollensak J. Myopic photorefractive keratectomy with excimer laser; oneyear followup. Ophthalmology. 1991;98:1156–63. doi: 10.1016/s0161-6420(91)32157-2. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton DR, Johnson RD, Lee N, Bourla N. Differences in the corneal biomechanical effects of surface ablation compared with laser in situ keratomileusis using a microkeratome or femtosecond laser. J Cataract Refract Surg. 2008;34:2049–56. doi: 10.1016/j.jcrs.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Fay J, Juthani V. Current trends in pain management after photorefractive and phototherapeutic keratectomy. Curr Opin Ophthalmol. 2015;26:255–9. doi: 10.1097/ICU.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 4.Blake CR, Cervantes-Castañeda RA, Macias-Rodríguez Y, Anzoulatous G, Anderson R, Chayet AS. Comparison of postoperative pain in patients following photorefractive keratectomy versus advanced surface ablation. J Cataract Refract Surg. 2005;31:1314–9. doi: 10.1016/j.jcrs.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Girgis R, Morris DS, Kotagiri A, Ramaesh K. Bilateral corneal scarring after LASIK and PRK in a patient with propensity to keloid scar formation. Eye (Lond) 2007;21:96–7. doi: 10.1038/sj.eye.6702180. [DOI] [PubMed] [Google Scholar]
- 6.Ambrósio R, Jr, Wilson S. LASIK vs LASEK vs PRK: Advantages and indications. Semin Ophthalmol. 2003;18:2–10. doi: 10.1076/soph.18.1.2.14074. [DOI] [PubMed] [Google Scholar]
- 7.Steinert RF, Bafna S. Surgical correction of moderate myopia: Which method should you choose II PRK and LASIK are the treatments of choice. Surv Ophthalmol. 1998;43:157–79. doi: 10.1016/s0039-6257(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 8.Stojanovic A, Nitter TA. Correlation between ultraviolet radiation level and the incidence of late-onset corneal haze after photorefractive keratectomy. J Cataract Refract Surg. 2001;27:404–10. doi: 10.1016/s0886-3350(00)00742-2. [DOI] [PubMed] [Google Scholar]
- 9.O'Brart DP, Lohmann CP, Klonos G, Corbett MC, Pollock WS, Kerr-Muir MG, et al. The effects of topical corticosteroids and plasmin inhibitors on refractive outcome, haze, and visual performance after photorefractive keratectomy. A prospective, randomized, observer-masked study. Ophthalmology. 1994;101:1565–74. doi: 10.1016/s0161-6420(94)38032-8. [DOI] [PubMed] [Google Scholar]
- 10.Cua IY, Pepose JS. Late corneal scarring after photorefractive keratectomy concurrent with development of systemic lupus erythematosus. J Refract Surg. 2002;18:750–2. doi: 10.3928/1081-597X-20021101-16. [DOI] [PubMed] [Google Scholar]
- 11.Kumar NR, Khamar P, Shetty R, Sharma A, Shetty N, Pahuja N, et al. Identification of novel predictive factors for post surgical corneal haze. Sci Rep. 2019;9:16980. doi: 10.1038/s41598-019-53123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiserman I, Sadi N, Mimouni M, Sela T, Munzer G, Levartovsky S. Corneal breakthrough haze after photorefractive keratectomy with mitomycin C: Incidence and risk factors. Cornea. 2017;36:961–6. doi: 10.1097/ICO.0000000000001231. [DOI] [PubMed] [Google Scholar]
- 13.Sy ME, Zhang L, Yeroushalmi A, Huang D, Hamilton DR. Effect of mitomycin-C on the variance in refractive outcomes after photorefractive keratectomy. J Cataract Refract Surg. 2014;40:1980–4. doi: 10.1016/j.jcrs.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Lacayo GO, 3rd, Majmudar PA. How and when to use mitomycin-C in refractive surgery. Curr Opin Ophthalmol. 2005;16:256–9. doi: 10.1097/01.icu.0000172830.41394.7c. [DOI] [PubMed] [Google Scholar]
- 15.Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–8. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santhiago MR, Netto MV, Wilson SE. Mitomycin C: Biological effects and use in refractive surgery. Cornea. 2012;31:311–21. doi: 10.1097/ICO.0b013e31821e429d. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Vick S, Chen Z, Chen J, Watsky MA. Effects of vitamin D receptor knockout and vitamin D deficiency on corneal epithelial wound healing and nerve density in diabetic mice. Diabetes. 2020;69:1042–51. doi: 10.2337/db19-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang BC, Foo RC, Lim EW, Tan MM, Nah GK, Thean LS, et al. Risk factors for early-onset corneal haze after photorefractive keratectomy in an Asian population: Outcomes from the Singapore Armed Forces corneal refractive surgery programme 2006 to 2013. J Cataract Refract Surg. 2016;42:710–6. doi: 10.1016/j.jcrs.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Fantes FE, Hanna KD, Waring GO, 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–75. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- 20.Boulze-Pankert M, Dariel R, Hoffart L. Corneal scheimpflug densitometry following photorefractive keratectomy in myopic eyes. J Refract Surg. 2016;32:788–91. doi: 10.3928/1081597X-20160720-02. [DOI] [PubMed] [Google Scholar]
- 21.Majmudar PA, Schallhorn SC, Cason JB, Donaldson KE, Kymionis GD, Shtein RM, et al. Mitomycin-C in corneal surface excimer laser ablation techniques: A report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:1085–95. doi: 10.1016/j.ophtha.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 22.The definition and classification of dry eye disease: Report of the Definition And Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 23.Chao C, Richdale K, Jalbert I, Doung K, Gokhale M. Non-invasive objective and contemporary methods for measuring ocular surface inflammation in soft contact lens wearers-A review. Cont Lens Anterior Eye. 2017;40:273–82. doi: 10.1016/j.clae.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128:94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Teramukai S, Kinoshita S. Meibomian glands and ocular surface inflammation. Ocul Surf. 2015;13:133–49. doi: 10.1016/j.jtos.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin Proc. 2010;85:752–8. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reins RY, Hanlon SD, Magadi S, McDermott AM. Effects of topically applied vitamin D during corneal wound healing. PLoS One. 2016;11:e0152889. doi: 10.1371/journal.pone.0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy ZZ, Hiscott P, Seitz B, Schlötzer-Schrehardt U, Süveges I, Naumann GO. Clinical and morphological response to UV-B irradiation after excimer laser photorefractive keratectomy. Surv Ophthalmol. 1997;42(Suppl 1):S64–76. doi: 10.1016/s0039-6257(97)80028-8. [DOI] [PubMed] [Google Scholar]
- 29.Shetty R, Dalal R, Nair AP, Khamar P, D'Souza S, Vaishnav R. Pain management after photorefractive keratectomy. J Cataract Refract Surg. 2019;45:972–6. doi: 10.1016/j.jcrs.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocularsurface. Ophthalmology. 2001;108:1230–5. doi: 10.1016/s0161-6420(01)00623-6. [DOI] [PubMed] [Google Scholar]
- 31.Salomao MQ, Wilson SE. Corneal molecular and cellular biology update for the refractive surgeon. J Refract Surg. 2009;25:459–66. doi: 10.3928/1081597x-20090422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marino GK, Santhiago MR, Torricelli AA, Santhanam A, Wilson SE. Corneal molecular and cellular biology for the refractive surgeon: The critical role of the epithelial basement membrane. J Refract Surg. 2016;32:118–25. doi: 10.3928/1081597X-20160105-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naroo SA, Shah S, Chatterjee A, Morgan P, Kapoor R, Rigby P. Patient motivation in PRK and contact lenses. In: Lass JH, editor. Advances in Corneal Research. Boston, MA: Springer; 1997. [Google Scholar]
- 34.Brancato R, Fiore T, Papucci L, Schiavone N, Formigli L, Orlandini SZ, et al. Concomitant effect of topical ubiquinone Q10 and vitamin E to prevent keratocyte apoptosis after excimer laser photoablation in rabbits. J Refract Surg. 2002;18:135–9. doi: 10.3928/1081-597X-20020301-06. [DOI] [PubMed] [Google Scholar]
- 35.Nejima R, Miyata K, Tanabe T, Okamoto F, Hiraoka T, Kiuchi T, et al. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2005;139:64–71. doi: 10.1016/j.ajo.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 37.Shetty R, Sethu S, Deshmukh R, Deshpande K, Ghosh A, Agrawal A, et al. Corneal dendritic cell density is associated with subbasal nerve plexus features, ocular surface disease index, and serum vitamin D in evaporative dry eye disease. Biomed Res Int. 2016;2016:4369750. doi: 10.1155/2016/4369750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang JS, Lee YP, Shin YJ. Vitamin D enhances the efficacy of topical artificial tears in patients with dry eye disease. Cornea. 2019;38:304–10. doi: 10.1097/ICO.0000000000001822. [DOI] [PubMed] [Google Scholar]
- 39.Nien CJ, Flynn KJ, Chang M, Brown D, Jester JV. Reducing peak corneal haze after photorefractive keratectomy in rabbits: Prednisolone acetate 100% versus cyclosporine A 005% J Cataract Refract Surg. 2011;37:937–44. doi: 10.1016/j.jcrs.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yulish M, Beiran I, Miller B, Pikkel J. Ascorbate prophylaxis with mitomycin-C for corneal haze after laser-assisted sub-epithelial keratectomy. Isr Med Assoc J. 2012;14:382–5. [PubMed] [Google Scholar]
- 41.Carones F, Fiore T, Brancato R. Mechanical vs. alcohol epithelial removal during photorefractive keratectomy. J Refract Surg. 1999;15:556–62. doi: 10.3928/1081-597X-19990901-08. [DOI] [PubMed] [Google Scholar]
- 42.Kaluzny BJ, Cieslinska I, Mosquera SA, Verma S. Single-step transepithelial PRK vs alcohol-assisted PRK in myopia and compound myopic astigmatism correction. Medicine (Baltimore) 2016;95:e1993. doi: 10.1097/MD.0000000000001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohac M, Biscevic A, Koncarevic M, Anticic M, Gabric N, Patel S. Comparison of Wavelight Allegretto Eye-Q and Schwind Amaris 750S excimer laser in treatment of high astigmatism. Graefes Arch Clin Exp Ophthalmol. 2014;252:1679–86. doi: 10.1007/s00417-014-2776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]