Dear Editor,
Olfactory dysfunction is a well-known complication of coronavirus disease-2019 (COVID-19) and includes quantitative (hyposmia or anosmia) and qualitative (parosmia or phantosmia) olfactory dysfunction. Parosmia is defined as alteration of olfactory perception in the presence of real olfactory stimulation. Patients with parosmia often describe perceiving the smell of something that is burned, foul or rotten (1). Few mechanistic and imaging studies have been performed to evaluate the pathophysiology of parosmia, and none in parosmia due to COVID-19. We have previously reported the findings on MRI of the olfactory bulb (2) and cortical metabolic patterns on PET/CT imaging in anosmia of COVID-19 (3). In this study, we assessed the findings with multimodality PET/CT and MRI in parosmia secondary to COVID-19.
We included a 28-year-old healthy right-handed woman with COVID-19, confirmed by polymerase chain reaction assay 6 months ago, which was complicated with anosmia. While anosmia was gradually improving, she developed parosmia, which has persisted for the past 3 months. For PET/CT imaging, the patient fasted for 6 hours before receiving intravenous 2-deoxy-2-[18F]-fluoro-D-glucose (18FDG, 4.6 MBq/kg). We performed the scan in a semi-darkened, noiseless, and odorless room, with the patient's eyes closed for 20 minutes. The brain PET/CT was performed with sequential TOF-PET/CT (Discovery 690 PET/CT, GE Healthcare). We performed MRI to assess the olfactory bulb and tract volume and signal changes. We used the PMOD Neuro Tool (PMOD Technologies Ltd., Version 4.2) to segment the cortex and the basal ganglia from the T1-weighted MRI.
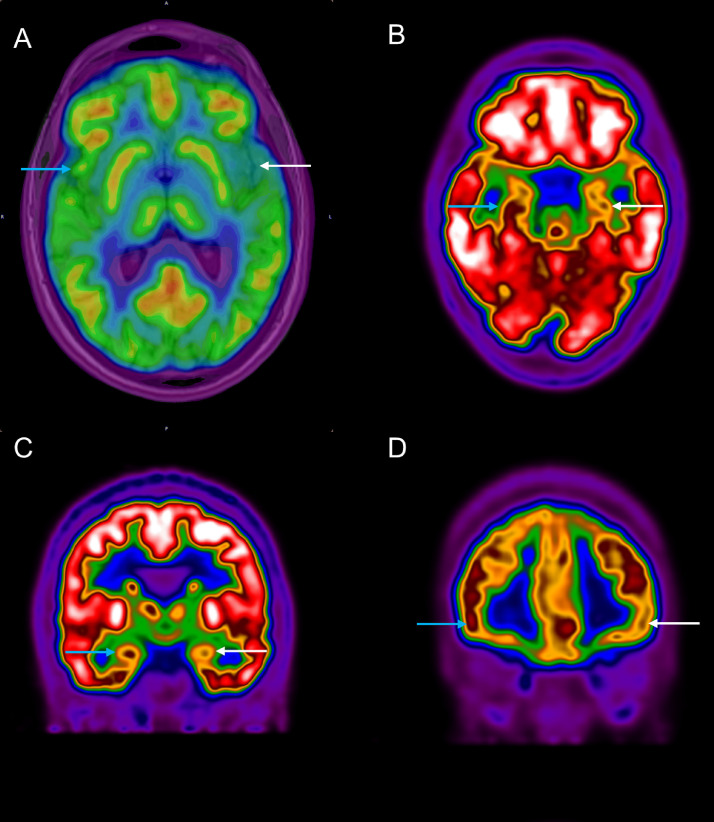
On MRI, the olfactory bulb and tract volumes were normal, and no signal changes were detected. There was a reduction in the maximal standardized uptake value (SUVmax) in the left insula, left inferior frontal gyrus, left hippocampus, and left amygdala compared with the contralateral side (Fig 1 , Table 1 ).
Figure 1.
18FDG-PET/CT in a 28-year-old woman with parosmia due to COVID-19. Axial (A, B) and coronal (C, D) planes of brain are shown. (A) On combined PET and MRI, the maximal standardized uptake value was lower in the left insular cortex (white arrow) compared with the right side (blue arrow). There was decreased 18FDG uptake in the left side (white arrows) compared with the right side (blue arrows) in hippocampus (b), amygdala (c), and inferior frontal gyrus (d). (Color version of figure is available online.)
Table 1.
Maximal Standardized Uptake Value (SUVmax) in the Olfactory Cortices in Parosmia of COVID-19
| Region | Side | SUVmax | Reduction (L vs. R) |
|---|---|---|---|
| Insular cortex | R | 5.6 | 23% |
| L | 4.3 | ||
| Hippocampus | R | 4.3 | 12% |
| L | 3.8 | ||
| Amygdala | R | 4.2 | 12% |
| L | 3.8 | ||
| Inferior frontal gyrus | R | 7.2 | 11% |
| L | 6.4 |
Two working hypotheses exist for parosmia—the peripheral hypothesis indicates incomplete regeneration of olfactory neurons while the central hypothesis implies malfunction of the olfactory centers in the brain (1). A reduction in the volume of primary olfactory centers, most importantly the olfactory bulb, has been reported in parosmia (4), nonetheless we did not find structural or metabolic abnormalities in this region in parosmia; findings that are consistent with our observations in anosmia of COVID-19 (3). Moreover, we did not detect structural or metabolic abnormalities in the secondary olfactory cortex, notably the pririform cortex.
In contrast to anosmia of COVID-19, where the metabolic activity of the left orbitofrontal cortex was reduced (3), we did not detect a change in the 18FDG uptake in this tertiary olfactory center in parosmia. We observed decreased 18FDG uptake in several other tertiary olfactory cortices, most prominently in the left insular cortex, compared to the contralateral side (Table 1). The insular cortex functions as an integrative center for multimodality convergence of inputs from the gustatory and olfactory cortices, and processes the odor quality (1). The reduction in metabolic activity of the left insular cortex in the present study is consistent with previous reports of structural remodeling of this region in parosmia (1). Nonetheless, we did not detect volume reduction on MRI in this cortical region in this patient with a 3-month history of parosmia. The relative hypometabolism in the left inferior frontal cortex, involved in odor identification and semantic association, and left hippocampus, involved in odor quality discrimination and olfactory memory processing (5), are consistent with previous reports of structural remodeling in these cortices in parosmia due to other etiologies (1). Last, we detected hypometabolism in the left amygdala that is involved in emotionally aversive response to odors (1).
To the best of our knowledge, this is the first report of combined PET/CT and MRI assessment of parosmia in COVID-19. Decreased metabolic activity without volume reduction in the tertiary olfactory cortex involved in the quality processing and affective response to odors in the present report suggests functional rather than structural impairment, and put emphasis on the central hypothesis in pathogenesis of parosmia. The observed changes might be a consequence of neuro-invasion by COVID-19 (6). Nonetheless, further studies are warranted to evaluate the mechanisms of parosmia in COVID-19.
Prior Presentations
None.
Author Contribution
A.Y., M.B., N.R., and M.K. have provided the case and images and M.K and S.H. have written the article.
Disclosures
The authors report no conflict of interest or funding sources.
References
- 1.Bitter T, Siegert F, Gudziol H. Gray matter alterations in parosmia. Neuroscience. 2011;177:177–182. doi: 10.1016/j.neuroscience.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Galougahi MK, Ghorbani J, Bakhshayeshkaram M. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karimi-Galougahi M, Yousefi-Koma A, Bakhshayeshkaram M. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rombaux P, Mouraux A, Bertrand B. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. 2006;116:436–439. doi: 10.1097/01.MLG.0000195291.36641.1E. [DOI] [PubMed] [Google Scholar]
- 5.Goodrich-Hunsaker NJ, Gilbert PE, Hopkins RO. The role of the human hippocampus in odor-place associative memory. Chem Senses. 2009;34:513–521. doi: 10.1093/chemse/bjp026. [DOI] [PubMed] [Google Scholar]
- 6.Butowt R, Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020;11:1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]