-
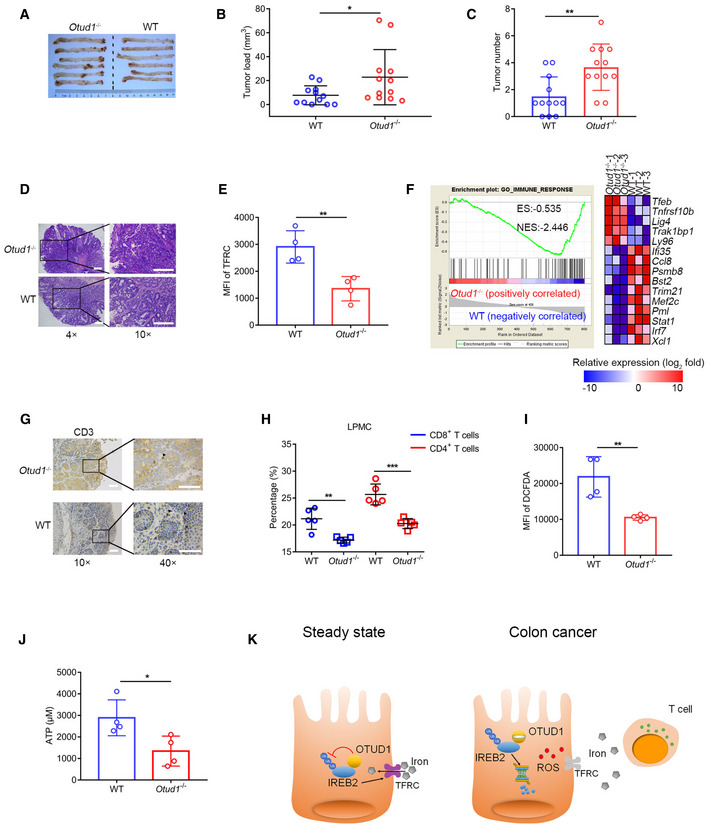
A
Representative pictures of colon tumors in AOM/DSS‐induced colitis‐associated cancer (CAC) model (n = 6 biological replicates).
-
B, C
The tumor load (B) or tumor number (C) in the whole colon was measured (n = 12 biological replicates, mean ± s.e.m., *P = 0.0431, **P = 0.003, two‐tailed unpaired Student’s t‐test).
-
D
Representative H&E (hematoxylin–eosin) staining pictures of colon tumors (the scale bars represent 1,000 μm).
-
E
Flow cytometric analysis of TFRC expression in intestinal epithelial cells (IECs) from wild‐type (WT) and Otud1
−/− mice treated with AOM/DSS. MFI, mean fluorescence intensity. (n = 4 biological replicates, mean ± s.e.m., **P = 0.0062, two‐tailed unpaired Student’s t‐test).
-
F
GSEA of genes expressed in colon tissues from wild‐type (WT) and Otud1
−/− mice treated with AOM/DSS (n = 3 biological replicates). ES, enrichment score; NES, normalized enrichment score.
-
G
Representative immunohistochemistry staining pictures of CD3+ T cells in colon tumors (the scale bars represent 400 μm).
-
H
Flow cytometric analysis of various T‐cell subsets in lamina propria mononuclear cells (LPMC) isolated from wild‐type (WT) and Otud1
−/− mice treated with AOM/DSS (n = 5 biological replicates, mean ± s.e.m., **P = 0.0025, ***P = 0.0005, two‐tailed unpaired Student’s t‐test).
-
I
Intracellular ROS levels in colon tissues from wild‐type (WT) or Otud1
−/− mice treated with AOM/DSS (n = 4 biological replicates, mean ± s.e.m., **P = 0.0072, two‐tailed unpaired Student’s t‐test) were detected by DCFDA staining. MFI, mean fluorescence intensity.
-
J
ATP level in tumor interstitial fluid (TIF) of mice treated with AOM/DSS (n = 4 biological replicates, mean ± s.e.m., *P = 0.0292, two‐tailed unpaired Student’s t‐test).
-
K
Model for the role of OTUD1 in iron absorption and tumor suppression. OTUD1 facilitates TFRC‐mediated iron absorption through stabilization of IREB2 under steady condition. However, during colorectal cancer development, downregulation of OTUD1 restricts iron absorption and promotes resistance to ferroptosis, thereby subverting host immune surveillance against cancer.