Figure 5.
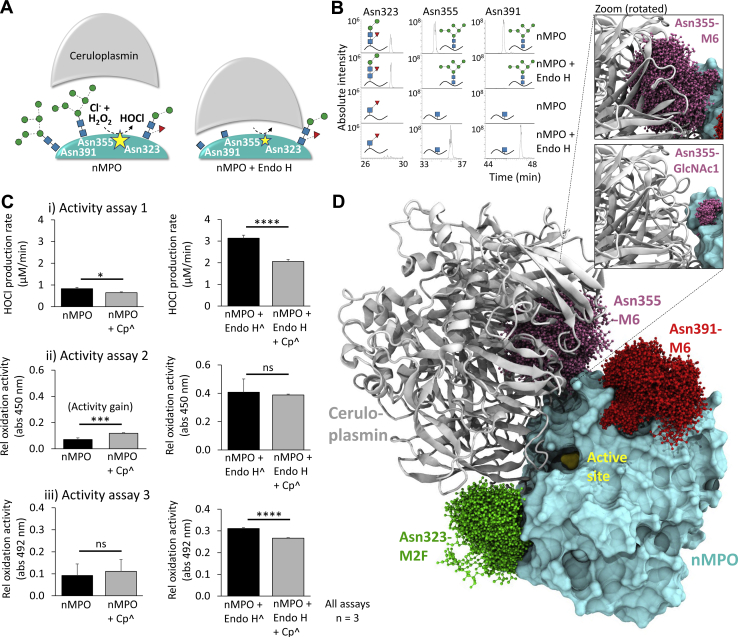
Hyper-truncated Asn355-glycans augment the ceruloplasmin-mediated MPO inhibition.A, schematics of the glycoform-dependent ceruloplasmin-based inhibition of Endo H-treated and untreated nMPO. Our data support that nMPO glycoforms carrying hyper-truncated GlcNAc signatures at Asn355 and Asn391 enable a closer contact to the natural MPO-inhibitor ceruloplasmin thereby sterically precluding an efficient substrate-product exchange to the catalytic site of nMPO as illustrated. Common glycans decorating Asn323, Asn355 and Asn391 in proximity to the MPO-ceruloplasmin interface and the HOCl-producing active site (yellow star) are portrayed. B, glycopeptide data (selected EICs) demonstrating complete conversion of Asn355-M6 and Asn391-M6 to GlcNAcβAsn upon Endo H-treatment (Fig. S7). The Endo H-insensitive Asn323-M2F was included as a control. C, the chlorination (i) and relative oxidation (ii–iii) levels of native nMPO (left graphs) and Endo H-treated nMPO (right) incubated with (gray bars) and without (black) serum ceruloplasmin (Cp) were determined in technical triplicates using activity assay 1-3, respectively (Table S9, D–F). The activity data of nMPO and Endo H-treated nMPO (black bars) already shown in Figure 4B, ii were included again to allow for a comparison to the activity data of the Cp-treated nMPO. ˆEndo H-and Cp-treated nMPO data were normalised based on Endo H and Cp only control data to enable comparison to untreated nMPO (for all assays, n = 3, technical replicates). D, MD data of Asn323-M2F (green), Asn355-M6 (magenta) and Asn391-M6 (red) modelled on a crystal structure of the ceruloplasmin–MPO complex (PDBID, 4EJX) demonstrated that Asn355-M6 and not Asn355-GlcNAc clashes with ceruloplasmin (see zoom). Data plotted as mean ± SD, ns, not significant (p ≥ 0.05), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001.