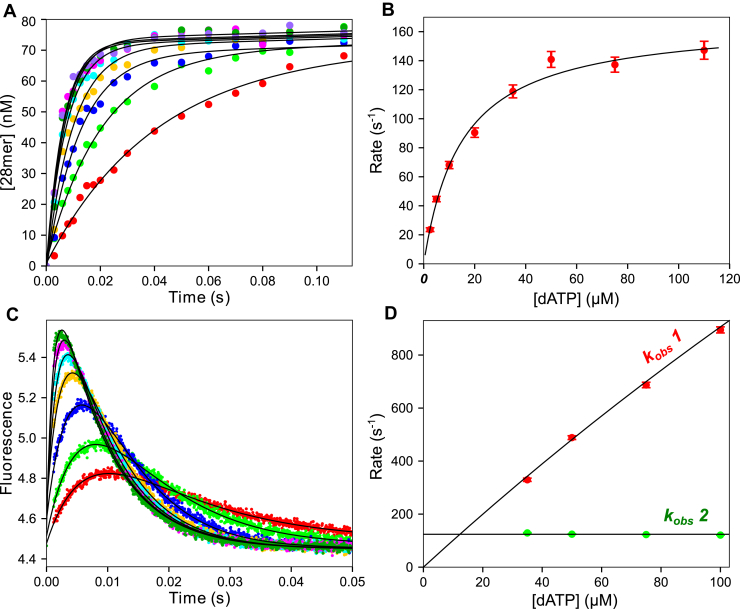
Figure 3.
Kinetics of dATP incorporation.A, quench flow pre-steady-state burst experiment. T7 DNA polymerase E514Cou (120 nM), BSA (0.1 mg/ml), thioredoxin (2.4 μM), and FAM-27/45 DNA (200 nM) were mixed with dATP (2.5–110 μM) and Mg2+ (12.5 mM) to start the reaction. Data at various dATP concentrations are shown as different colors, fit to a burst equation (black line) at each concentration. B, rate versus dATP concentration. Rates are from the fit of the exponential phase in (A) and are shown fit to a hyperbola (black line), estimating the maximum rate, kpol = 210 ± 15 s−1 and Kd,app =15.5 ± 2 μM. C, stopped flow dATP incorporation. T7 DNA polymerase E514Cou (750 nM), thioredoxin (15 μM), and DNA 27/45 (1 μM) were mixed with dATP (5–100 μM) and Mg2+ (12.5 mM) to start the reaction. Data at each dATP concentration were fit to a double exponential function (black lines). D, rate versus dATP concentration for stopped flow fluorescence. Rates are from the data in (C). The fast phase data (red points, kobs 1) were fit to a hyperbola to estimate the maximum rate of the conformational change (k2), 7800 ± 3400 s−1. The slow phase data (green points, kobs 2) were fit to a line, setting a lower limit on the maximum rate of the isomerization following chemistry (k4) of 126 ± 4 s−1.