Fig. 2. In vitro synthesis of licensed conjugate vaccine carrier proteins.
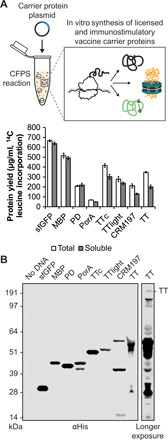
(A) All four carrier proteins used in approved conjugate vaccines were synthesized solubly in vitro, as measured via 14C-leucine incorporation. These include H. influenzae PD, the N. meningitidis porin protein (PorA), and genetically detoxified variants of the C. diphtheriae toxin (CRM197) and the C. tetani toxin (TT). Additional immunostimulatory carriers, including E. coli MBP and the fragment C (TTc) and light chain (TTlight) domains of TT, were also synthesized solubly. Values represent means and error bars represent SDs of biological replicates (n = 3). (B) Full-length product was observed for all proteins tested via Western blot. Different exposures are indicated with solid lines. Molecular weight ladder is shown at left. αHis, anti–hexa-histidine antibody.
