Fig. 3. Reproducible glycosylation of proteins with FtO-PS in iVAX.
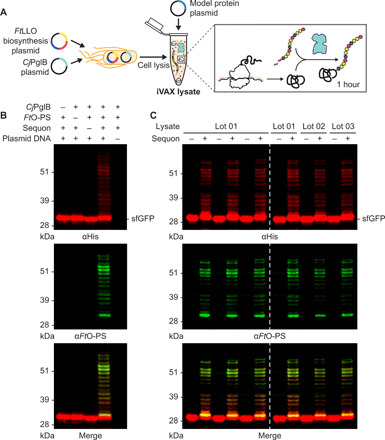
(A) iVAX lysates were prepared from cells expressing CjPglB and the biosynthetic pathway encoding FtO-PS. (B) Glycosylation of sfGFP217-DQNAT with FtO-PS was only observed when CjPglB, FtO-PS, and the preferred DQNAT glycosylation sequence (sequon) were present in the reaction (lane 3). When plasmid DNA was omitted, sfGFP217-DQNAT synthesis was not observed. (C) Biological replicates of iVAX reactions producing sfGFP217-DQNAT using the same lot (left) or different lots (right) of iVAX lysates demonstrated reproducibility of reactions and lysate preparation. Top panels show signal from probing with αHis to detect the carrier protein, middle panels show signal from probing with commercial anti–FtO-PS antibody (αFtO-PS), and bottom panels show αHis and αFtO-PS signals merged. Images are representative of at least three biological replicates. Dashed lines indicate that samples are from nonadjacent lanes of the same blot with the same exposure. Molecular weight ladders are shown at the left of each image.
