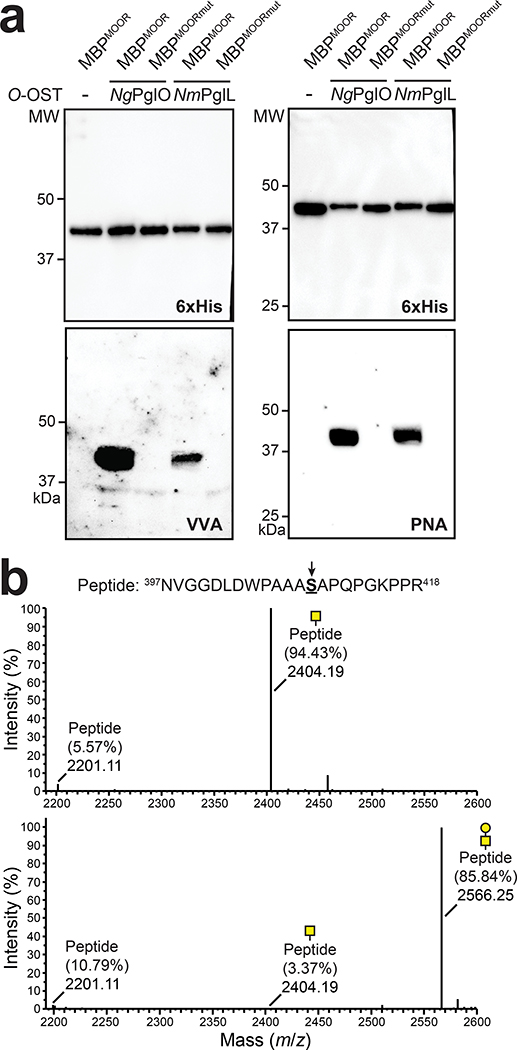
Figure 2. Biosynthesis of O-glycoproteins bearing Tn and T antigens.
(a) Immunoblot analysis of acceptor proteins purified from CLM25 (W3110 ΔwecA ΔwaaL) cells co-transformed with pOG-Tn (left panels) or pOG-T (right panels) without an O-OST (−), pOG-Tn-NgPglO, or pOG-Tn-NmPglL along with pEXT-spDsbA-MBPMOOR or pEXT-spDsbA-MBPMOORmut as indicated. Absence of O-OST or mutation of acceptor serine to glycine in MBPMOORmut served as controls. Blots were probed with anti-hexa-histidine antibody (6xHis) to detect acceptor proteins and either VVA or PNA lectin to detect the Tn or T antigen, respectively. Molecular weight (MW) markers are indicated on the left. Results are representative of at least three biological replicates. See Supplementary Fig. 1 for uncropped versions of the images. (b) Nano-LC-MS/MS analysis of purified acceptor protein generated by CLM25 cells carrying plasmid pOG-Tn-NgPglO (top spectrum) or pOG-T-NgPglO (bottom spectrum) and pEXT-spDsbA-MBPMOOR. Sequence coverage of 88% and 75% was obtained for glycosylated MBPMOOR with Tn and T antigens, respectively, in the analysis. Spectrum for Tn glycoform reveals a dominant species (94% abundance) corresponding to peptide fragment bearing a single HexNAc and a less abundant (6%) aglycosylated species. Spectrum for T glycoform reveals a dominant species (86% abundance) corresponding to peptide fragment bearing a single HexHexNAc as well as two minor species bearing a single HexNAc and no modification (3% and 11% abundance, respectively). Sequence of detected peptide is shown at top with arrow denoting modified serine (bold underline) as determined by EThcD fragmentation analysis.