Abstract
Background
Different strategies can be used to counteract coagulation of extracorporeal systems. Systemic anticoagulation is most widely used in routine clinical practice, but can be contraindicated in specific settings. The Solacea™ dialyser, containing the asymmetric triacetate membrane, claims improved biocompatibility, which should result in decreased tendency for coagulation. We quantified the performance of the Solacea™ versus the FX800CORDIAX dialyser regarding resistance to fibre blocking as assessed by micro-computed tomography (CT).
Methods
This cross-over study with four arms randomized consecutively 10 maintenance haemodialysis patients to a 4-h post-dilution haemodiafiltration session at midweek, using either Solacea™ 19 H or FX800CORDIAX, with either regular or half dose of anticoagulation (EC2017/1459-NCT03820401). Dialyser fibre blocking was visualized in the dialyser outlet potting using a 3D CT scanning technique on micrometre resolution. Extraction ratios of middle molecules [myoglobin, lambda and kappa free light chains (FLCs)] were determined.
Results
The relative number of open fibres post-dialysis was lower in FX800CORDIAX versus Solacea™ dialyser, and this was irrespective of the anticoagulation dose used or the threshold for counting open fibres. Extraction ratios of FLCs were not different at regular anticoagulation between Solacea™ and FX800CORDIAX (21% ± 4% for kappa and 32% ± 8% for lambda with Solacea™ versus 23% ± 7% and 38% ± 6% for FX800CORDIAX), but were superior with the Solacea™ (34% ± 12% versus 22% ± 8% with FX800CORDIAX; P = 0.02) for myoglobin in case of halving anticoagulation dose. No clinically relevant albumin loss was detected.
Conclusions
The Solacea™ dialyser seems to be promising for use in conditions where systemic anticoagulation is contraindicated, as even under conditions of low systemic anticoagulation, virtually no signs of fibre blocking could be observed using the sensitive micro-CT scanning technique. This finding is in line with its presumed good performance in terms of biocompatibility.
Keywords: anticoagulation, clotting, haemodialysis, micro-CT scan, polymers
INTRODUCTION
The retention of uraemic toxins in patients with end-stage kidney disease (ESKD) has been associated with inflammation and increased cardiovascular risk. The removal of middle molecules, such as β2-microglobulin (β2M), and of protein-bound toxins, such as indoxylsulphate, is rather poor with conventional low-flux dialysis, whereas these contribute probably most to uraemic toxicity [1, 2]. In an attempt to reduce inflammation and thus cardiovascular toxicity, the use of haemodiafiltration (HDF) or of more open membranes has been promoted to enhance clearance of middle molecular weight substances during dialysis [3].
Membranes used in online HDF treatment must have high permeability while still keeping albumin (Alb) leakage to a minimum. Polysulfone and polyethersulfone membranes have generally been used to satisfy these requirements. However, these membranes are sometimes associated with hypersensitivity reactions, attributed to the use of additives such as polyvinylpyrrolidone to enhance hydrophilic properties [4]. As the asymmetric triacetate (ATA™) membrane is manufactured without hydrophilization agents, it was found to have a lower risk of hypersensitivity, and less decrease in platelets. It has a dense skin layer on the internal surface at blood side, and an external support layer with large pores, maintaining high permeability and filtration performance [4].
Coagulation within the dialyser membrane fibres is an obvious biological sign of bio-incompatibility [5]. To avoid clotting during extracorporeal treatment, an anticoagulant is added to the circuit, resulting in an increased risk for bleeding complications. In clinical practice, it regularly happens that patients needing extracorporeal treatment have a relative contraindication for systemic anticoagulation, for example, because of active bleeding (gastrointestinal conditions) or with increased bleeding risk (peri-operative period). Disposing of membranes with low procoagulant properties, so that the need for systemic anticoagulation can be minimized, can thus be a true clinical advantage.
In addition, there is evidence that a substantial number of fibres can become blocked before this is reflected in routinely observed parameters, or in termination of the dialysis session [6]. Little is known about the impact of such subclinical clotting on dialyser performance in terms of solute clearance.
Membrane clogging due to deposition of proteins and red blood cells on the dialysis membrane may influence both the diffusive and convective transport characteristics of the dialyser membrane before leading to complete dialyser clotting [7–9]. Recently, a method was described to objectively count the number of blocked fibres inside a dialyser using a micro-computed tomography (CT) scanning technique [6]. This method can thus also be used to assess the resistance of the dialyser to clotting and to evaluate the impact of subclinical fibre blocking on solute removal and thus the performance of a dialyser during a dialysis session.
The aim of this randomized cross-over study was to objectively quantify the performance of the Solacea™ dialyser containing the ATA™ membrane, and compare it for different performance outcomes with those of the FX800CORDIAX dialyser containing a polysulfone membrane. To more intensely activate the coagulation cascade, dialysis was performed once with regular and once with only half of the regular anticoagulation dose. We used resistance to fibre blocking as assessed by micro-CT as primary outcome, and dialyser clearances of small water soluble solutes and middle molecules, and Alb loss, as secondary outcome.
MATERIALS AND METHODS
Patients
Power analysis was based on data from a previously performed cross-over study in patients dialysed with two different types of dialyser [10]. Using the relative number of patent fibres as primary outcome, power was 69% (α = 0.05) including only six patients. Therefore, this single-centre randomized cross-over study with four treatment regimens included 10 consecutive stable chronic haemodialysis (HD) patients [aged 65.9 ± 16.3 years, 15.2 (9.6–25.9) months on dialysis, and all male]. Patients were eligible when they had experienced stable dialysis sessions during the last 4 weeks, and had no known coagulation disorder, active inflammation or malignancy.
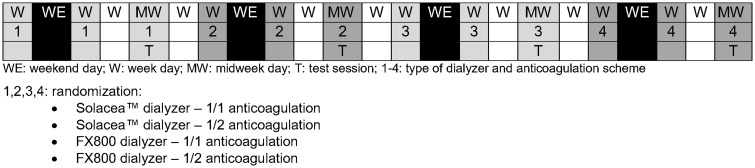
Since test sessions were scheduled at midweek with a washout period of 1 week, test sessions can be considered as independent and sequence order impact can be accepted to be absent. Furthermore, patients are serving as their own control such that simple randomization is allowed. At inclusion, patients were sequentially numbered (by the including nephrologist) for further simple randomization (www.randomization.com) (by the investigator) (Figure 1).
FIGURE 1.
Patient flow.
Double-needle vascular access was achieved through a native arteriovenous fistula (n = 7) or a well-functioning double-lumen tunnelled central venous catheter, either Hemostar® 14.5 F (n = 2) (Bard, Salt Lake City, UT, USA) or Palindrome™ 14.5 F (n = 1) (Medtronic, Minneapolis, MN, USA). Regular treatment of these patients was post-dilution HDF with high-flux dialysers FX800CORDIAX (n = 9) (Fresenius Medical Care, Bad Homburg, Germany) or Polyflux 170 H (n = 1) (Baxter, Deerfield, IL, USA).
The protocol was approved by the institutional research committee (Ethical Committee—Ghent University Hospital, EC 2017/1459-B670201734230—March 2018), and registered as part of a larger study in www.ClinicalTrials.gov (NCT03820401). Written informed consent was obtained from all included patients.
Dialysis and anticoagulation
In the study protocol, each patient was dialysed for 240 min in four different regimens, randomly using two different dialysers and two different anticoagulation schemes. All study sessions were performed at midweek in the dialysis unit of the Ghent University Hospital (Belgium) in the period 2–29 March 2018. The two dialysers investigated were the ATA™ Solacea™ 19 H (Nipro, Osaka, Japan) and the Helixone polysulfone FX800CORDIAX (Fresenius Medical Care, Bad Homburg, Germany). For optimal comparison, surface areas of both dialysers were selected to be approximately identical (Table 1).
Table 1.
Properties of the different dialyzers
| Sieving coefficient |
||||||||
|---|---|---|---|---|---|---|---|---|
| Membrane | Area (m²) | K UF (mL/h/mmHg) | D fibre (µm) | d (µm) | Sterilization technique | β2M | Myoglobin | |
| Solacea TM -19H | ATATM | 1.9 | 72 | 200 | 25 | gamma | 0.85 | 0.80 |
| FX 800CORDIAX | PS | 2.0 | 64 | 210 | 35 | INLINE steam | 0.90 | 0.50 |
K UF, ultrafiltration coefficient; Dfibre, fibre diameter; d, membrane thickness.
Patients received their regular brand of low-molecular-weight heparin (Tinzaparin, Leo Pharma, Belgium) anticoagulation at the beginning of the dialysis session, and this randomly is either at their regular dose (full dose 1/1) or at only 50% of their regular dose (half dose 1/2).
All test sessions were performed on a 5008 dialysis machine (Fresenius Medical Care, Bad Homburg, Germany) with blood flow at 300 mL/min and dialysate flow at 500 mL/min in post-dilution HDF mode. Substitution flow was set 25% of blood flow (i.e. 75 mL/min). Ultrafiltration rates were set according to the patient’s interdialytic weight gain and clinical status.
In this way, each patient served as his own control and was randomized over four test sessions at midweek (Figure 2). Each experimental session was preceded with two wash-in sessions with the same type of dialyser to be used in the experimental dialysis, but always with full regular anticoagulant dose.
FIGURE 2.
Test protocol.
Blood sampling, laboratory and calculations
During the four experimental midweek sessions, blood was sampled from the arterial and venous blood lines, and spent dialysate was sampled from the outlet line, all at 60 min after the dialysis start. Blood samples were immediately centrifuged and serum and dialysate were stored at −80°C until batch analysis in the routine laboratory of the Ghent University Hospital.
Concentrations of the small water-soluble solute urea were determined by routine analysis, while those of the middle molecules kappa and lambda free light chains (FLCs), and myoglobin were determined by nephelometry. Colorimetric bromocresol green assay was used to determine Alb concentrations.
Extraction ratios (ER) were calculated from the arterial and venous plasma concentrations (Carterial and Cvenous), accounting for haemoconcentration (based on Alb levels) for the middle molecules:
| (1) |
Alb loss during the dialysis session was quantified from adsorption and transmembrane loss in the dialysate. Adsorption of Alb and the middle molecules was calculated from the mass balance in the dialyser, accounting for haemoconcentration (using ultrafiltration rates):
| (2) |
where QB is bloodflow, QD is dialysate flow and QUF is ultrafiltration.
Micro-CT scanning and coagulation quantification
To quantify the primary outcome measure, i.e. incidence of fibre blocking, of the dialysers after 4 h HDF, dialysers were scanned with a reference non-invasive micro-CT scanning technique [6]. In brief, at the end of the dialysis session, a standard rinsing procedure of the haemodialyser was performed using exactly 300 mL of rinsing solution. Next, the haemodialyser was dried for 24 h using continuous positive pressure ventilation, simultaneously in blood and dialysate compartment. Dialyser fibre blocking was visualized in the dialyser outlet potting using a 3D CT scanning technique on micrometre resolution, as previously described [6].
For this study, three different thresholds were used to define the surface area of an open fibre: i.e. 50, 70 and 90% of the cross-section of a non-used fibre. Comparing the number of non-blocked fibres in the tested dialyser with the total number of fibres as measured in three non-used dialyser samples provided an objective estimate of the percentage of fibre blocking.
Statistical analysis
Statistical analyses were performed using SPSS version 24 (SPSS Inc, Chicago, IL, USA). Continuous variables were summarized as mean ± SD, and median value with interquartile range. To compare different related variables, ANOVA tests were performed with Tukey’s HSD post hoc test (normal distributions) or Friedman tests with Wilcoxon post hoc test (no normal distributions). A general linear model was used to assess eventual interaction between membrane and anticoagulation dose effects.
RESULTS
Relevant demographic and clinical data of the patient population at baseline are summarized in Table 2. There were no patient dropouts during the experimental period, all flow settings were maintained according to the protocol, and no adverse events were recorded. Table 3 shows the dialysis durations and the ultrafiltration rates in the four test sessions.
Table 2.
Demographic and clinical data of the patient population at baseline
| Gender (male/female) | 10/0 |
| Age, years | 65.9 ± 16.3 |
| BMI, kg/m² | 27.7 ± 7.0 |
| Dialysis vintage, months | 15.2 (9.6–25.9) |
| Renal disease | Nephroangiosclerosis (n = 3); diabetic nephropathy (n = 2); IgA nephropathy (n = 1); focal segmental glomerulosclerosis (n = 1); retroperitoneal fibrosis (n = 1); renal cell carcinoma (n = 1); Alport (n = 1) |
| Regular anticoagulation dose | Tinzaparin 3500 (n = 3); Tinzaparin 4500 (n = 7) |
| Anticoagulation/body weight (U/kg) | 49 (47–61) |
| Platelet inhibitors | Acetylsalicylic acid 80 mg (n = 5) |
| Hb (g/dL) | 10.7 ± 1.2 |
| Platelets count (10³/µL) | 217 ± 46 |
| aPTT (s) | 38.7 ± 5.9 |
| INR (−) | 1.0 ± 0.1 |
| CRP (mg/L) | 9.3 (6.4–12.0) |
Values are expressed as mean ± SD or median (interquartile range) unless otherwise specified. BMI, body mass index; IgA, immunoglobulin A; Hb, haemoglobin; aPTT, activated partial thromboplastin time; INR, international normalized ratio; CRP, C-reactive protein.
Table 3.
Characteristics of the dialysis sessions in the different experimental settings
| Solacea_1/1 | Solacea_1/2 | FX800CORDIAX_1/1 | FX800CORDIAX_1/2 | |
|---|---|---|---|---|
| Dialysis duration, min | 241 ± 2 | 240 ± 4 | 241 ± 2 | 241 ± 3 |
| V UF, mL | 1650 (750–2175) | 1695 (1500–2375) | 1800 (1200–2325) | 1700 (1350–2375) |
| Total convective volume, L | 17.3 (17.3–17.5) | 17.4 (17.4–17.7) | 17.4 (17.4–17.6) | 17.2 (17.1–17.6) |
Values are expressed as mean ± SD or median (25–75%); VUF, ultrafiltration volume over the session.
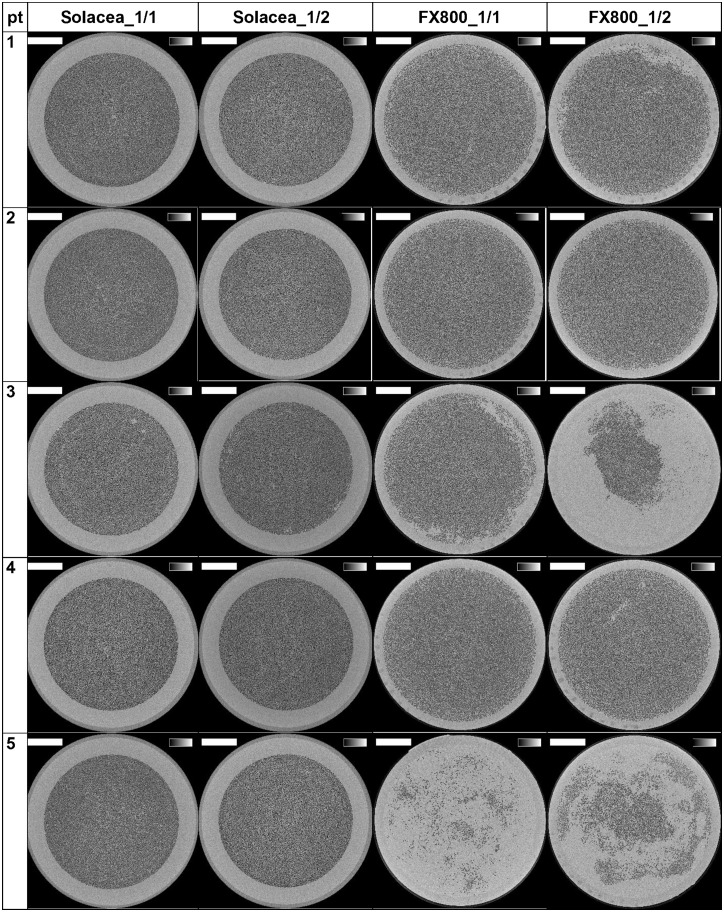
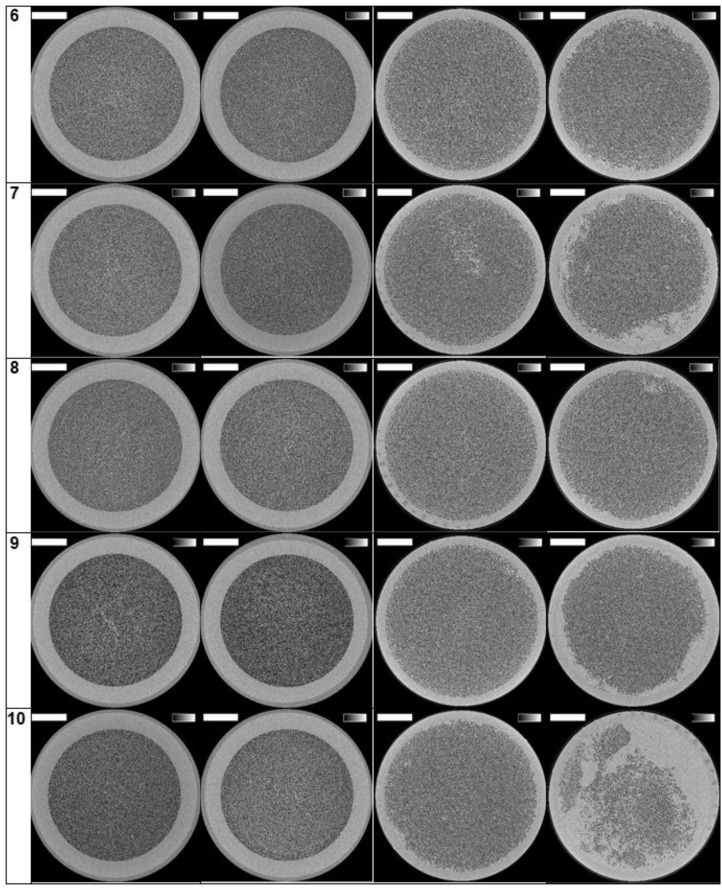
The reconstructed images of the cross-sections halfway the outlet potting are presented in Figure 3 for the 10 patients and for the four experimental dialysis sessions. The lumina of open fibres are visualized as black dots.
FIGURE 3.
Cross-sections halfway the potting in 10 patients and four tested settings. The grey scale range is from 0 to 0.5/cm and the scale bar denotes 10 mm.
The number of open fibres in the three non-used Solacea™ and FX800CORDIAX dialyser samples was 12 087 ± 4 and 13 051 ± 1, respectively, indicating high consistency in the number of fibres in non-used dialysers for both dialyser types.
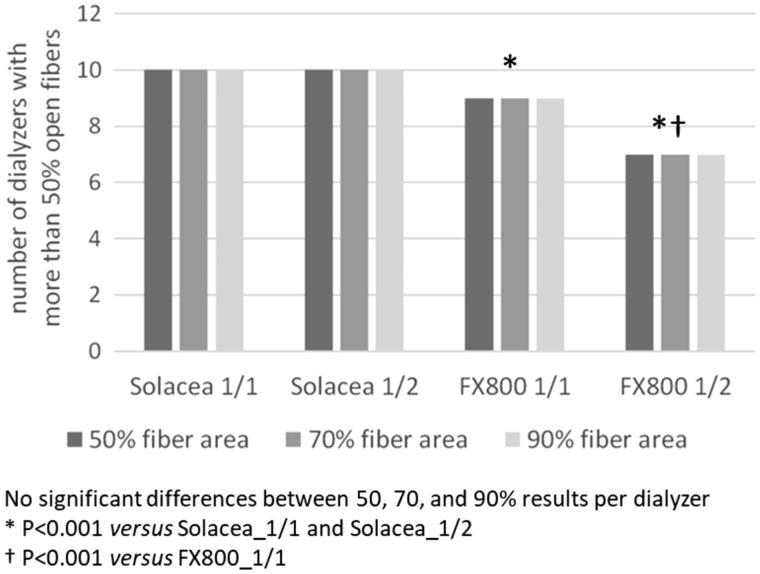
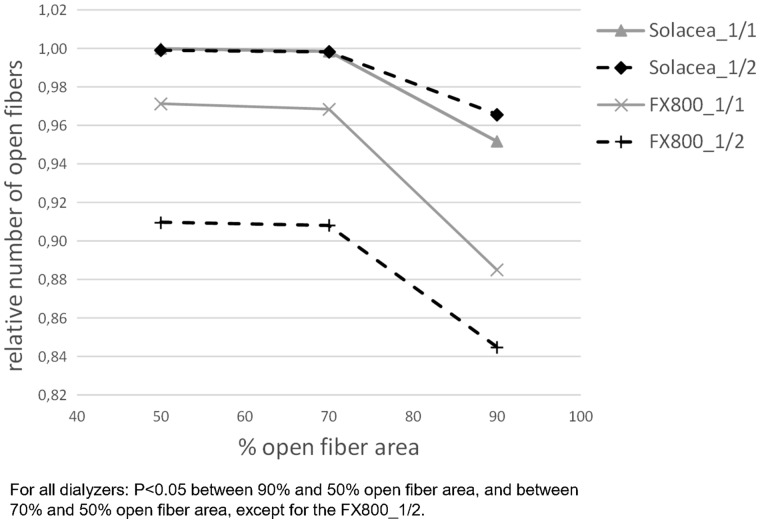
For the four test sessions, the number of dialysers with >50% open fibres are presented in Figure 4 for the different thresholds of considering fibres as being open (i.e. 50, 70 and 90% open fibre area). The numbers of open fibres relative to the number in non-used dialysers are given in Table 4. For a fixed anticoagulation protocol (either 1/1 or 1/2), irrespective of the considered threshold for counting open fibres, the relative number of patent fibres was lower in FX800CORDIAX versus Solacea™ dialyser. Accounting for 50 or 70% open area, halving anticoagulation resulted in relatively less open fibres as compared with full anticoagulation in the FX800CORDIAX, while no differences were seen in the Solacea™ dialyser.
FIGURE 4.
Number of dialysers with >50% of fibres considered as open according to the criteria of 50, 70 or 90% of single fibre area being free of clotting.
Table 4.
Relative number of open fibres in the four tested dialysers versus non-used dialysers for the thresholds of 50, 70 and 90% open fibre area
| Solacea_1/1, median (25–75%); (min–max) | Solacea_1/2, median (25–75%); (min–max) | FX800CORDIAX_1/1, median (25–75%); (min–max) | FX800CORDIAX_1/2, median (25–75%); (min–max) | Friedman, P-value | |
|---|---|---|---|---|---|
| 50% open area | 1.00 (1.00–1.00); (1.00–1.00) | 1.00 (1.00–1.00); (0.99–1.00)† | 0.97 (0.97–0.99); (0.16–1.00)†,‡ | 0.91 (0.57–0.97); (0.29–0.99)†,‡,* | <0.001 |
| 70% open area | 1.00 (1.00–1.00); (1.00–1.00) | 1.00 (0.99–1.00); (0.98–1.00) | 0.97 (0.97–0.99); (0.15–0.99)†,‡ | 0.91 (0.56–0.97); (0.28–0.99)†,‡,* | <0.001 |
| 90% open area | 0.95 (0.94–0.99); (0.88–1.00) | 0.97 (0.95–0.98); (0.88–1.00) | 0.89 (0.85–0.91); (0.10–0.93)†,‡ | 0.84 (0.40–0.89); (0.20–0.92)†,‡ | <0.001 |
P < 0.05 versus Solacea_1/1; ‡P < 0.05 versus Solacea_1/2; *P < 0.05 versus FX800CORDIAX_1/1.
In the general linear model, membrane type [Solacea™ versus FX800CORDIAX, β = −0.205, 95% confidence interval (CI) −0.39 to −0.019; P = 0.03] but not anticoagulation dose (full versus half, β = 0.101, 95% CI −0.085 to –0.286; P = 0.28) influenced the number of open fibres at 50%. There was no interaction between membrane type and anticoagulation dose on the effect on the number of open fibres.
Differences in clotting in both dialysers are also visualized in Figure 5. We observe that, overall, Solacea™ shows more open fibres versus FX800CORDIAX. Also, there is clearly a more substantial drop in number of fibres considered as open when the criterion shifts from 70% to 90% open fibre area. This indicates that the fibres of Solacea™ are resistant to even small degrees of fibre blocking during dialysis.
FIGURE 5.
Relative number of fibres considered as open according to different decision criteria of the percentage of fibre area free of clotting.
Concentrations of urea, Alb, myoglobin, and kappa and lambda FLCs as measured in blood sampled from the arterial and venous dialysis blood line, and from the spent dialysate line, are shown in Table 5. Alb concentrations in the spent dialysate were below the limit of detection (LOD), and no differences were found between the arterial and venous mass flow of Alb, suggesting the absence of Alb adsorption. While negligible adsorption onto the dialyser membrane was found for myoglobin, a substantial but comparable adsorption was found for kappa and lambda FLCs for both membranes (Table 6).
Table 5.
Uraemic toxin and Alb concentrations in blood and dialysate in different experimental settings
| Solacea_1/1 | Solacea_1/2 | FX800CORDIAX_1/1 | FX800CORDIAX_1/2 | |
|---|---|---|---|---|
| Urea, mg/dL | ||||
| Arterial | 65.3 ± 19.4 | 61.5 ± 25.7 | 63.5 ± 23.7 | 60.2 ± 26.3 |
| Venous | 6.0 ± 2.2 | 8.1 ± 7.2 | 5.7 ± 1.7 | 5.9 ± 2.0 |
| Dialysate | 29.2 ± 11.1 | 29.5 ± 13.8 | 29.0 ± 9.6 | 28.8 ± 11.5 |
| Alb (g/L) | ||||
| Arterial | 38.1 ± 4.4 | 38.7 ± 3.5 | 38.1 ± 4.3 | 38.0 ± 3.6 |
| Venous | 39.3 ± 4.8 | 41.0 ± 11.2 | 39.0 ± 4.8 | 39.4 ± 4.8 |
| Dialysate | LOD | LOD | LOD | LOD |
| Myoglobin (µg/L) | ||||
| Arterial | 129 ± 70 | 103 ± 45 | 149 ± 95 | 118 ± 66 |
| Venous | 91.5 ± 54.8 | 69.8 ± 32.0 | 113 ± 69 | 94.9 ± 51.1 |
| Dialysate | 26.9 ± 3.8 | 26.6 ± 2.3 | 28.5 ± 7.7 | 26.0 ± 4.3 |
| Kappa FLC, mg/L | ||||
| Arterial | 79.4 ± 40.1 | 84.2 ± 36.5 | 78.1 ± 39.8 | 70.0 ± 36.6 |
| Venous | 64.4 ± 31.5 | 64.9 ± 29.4 | 61.6 ± 34.2 | 55.5 ± 34.1 |
| Dialysate | 2.6 ± 1.7 | 2.8 ± 1.5 | 2.4 ± 1.9 | 2.6 ± 2.0 |
| Lambda FLC, mg/L | ||||
| Arterial | 102 ± 45 | 110 ± 45 | 90.0 ± 56.8 | 90.5 ± 43.2 |
| Venous | 69.6 ± 26.9 | 71.2 ± 27.6 | 67.7 ± 36.4 | 59.8 ± 36.4 |
| Dialysate | 3.1 ± 1.7 | 3.2 ± 1.3 | 2.6 ± 1.8 | 3.0 ± 2.4 |
Values are expressed as mean ± SD. LOD <4 g/L.
Table 6.
Membrane adsorption and extraction ratios
| P-value |
|||||||
|---|---|---|---|---|---|---|---|
| Solacea_1/1 | Solacea_1/2 | FX800CORDIAX_1/1 | FX800CORDIAX_1/2 | ANOVA | t-testa | t-testb | |
| Membrane adsorption (g/dialysis) | |||||||
| Kappa FLC | 0.89 ± 0.64 | 1.18 ± 0.67 | 0.84 ± 0.99 | 0.84 ± 0.64 | 0.71 | 0.40 | 0.40 |
| Lambda FLC | 2.07 ± 1.36 | 2.57 ± 1.32 | 2.38 ± 1.20 | 1.96 ± 1.45 | 0.73 | 0.41 | 0.61 |
| Extraction ratio (%) | |||||||
| Urea | 91 ± 2 | 87 ± 8 | 91 ± 2 | 89 ± 4 | 0.31 | 0.47 | 0.10 |
| Myoglobin | 32 ± 10 | 34 ± 12 | 24 ± 8 | 22 ± 8* | 0.02 | 0.01 | 0.97 |
| Kappa FLC | 21 ± 4 | 25 ± 13 | 23 ± 7 | 25 ± 6 | 0.63 | 0.52 | 0.28 |
| Lambda FLC | 32 ± 8 | 37 ± 8 | 38 ± 6 | 38 ± 12 | 0.46 | 0.37 | 0.27 |
Solacea versus FX800CORDIAX.
Full anticoagulation versus half-dose anticoagulation.
P < 0.05 versus Solacea_1/2.
Table 6 also shows the extraction ratios of urea and the middle molecules for the four dialysis scenarios at 60 min after dialysis start. For the Solacea™ with standard anticoagulation, extraction ratios were 21 ± 4% (kappa FLC), 32 ± 8% (lambda FLC) and 32 ± 10% (myoglobin), which were not different from those with the FX800CORDIAX. With half anticoagulation, however, extraction ratios for myoglobin were superior with the Solacea™ (34 ± 12% versus 22 ± 8% with FX800CORDIAX; P = 0.019). In general, irrespective of the anticoagulation dose, myoglobin extraction ratios were higher with Solacea™ versus FX800CORDIAX, while in both dialyser types no impact of anticoagulation dose on extraction ratio could be observed 60 min after dialysis start.
DISCUSSION
The present randomized cross-over study investigated the performance with respect to fibre blocking, middle molecule removal and Alb loss of the Solacea™ and FX800CORDIAX dialysers, and using either 100% or 50% of the regular anticoagulation dose.
Our main finding is that Solacea™ outperforms FX800CORDIAX in avoiding clotting as expressed by the relative number of open fibres at the end of the dialysis session, and this is even more so when the coagulation cascade is challenged by using only half the regular dose of anticoagulation. We observed a decrease in extraction ratio of middle molecules represented by myoglobin due to fibre blocking during dialysis with the FX800CORDIAX, a decrease in efficiency that can go unnoticed using current monitoring techniques. Both membranes resulted in comparable extraction ratios of Kappa and Lambda FLCs. There is practically no Alb loss when using the Solacea™ or the FX800CORDIAX dialyser.
Coagulation within the extracorporeal circuit can result in a precocious termination of the dialysis treatment, or worse, loss of extracorporeal circuit and the blood contained in it. To avoid this, many dialysis units have a policy of liberal use of anticoagulant agents, which might, however, result in more bleeding episodes [11]. In conditions where extracorporeal treatment is necessary but systemic anticoagulation should be avoided, e.g. with active bleeding or increased bleeding risk, it is important to select a dialyser that activates the coagulation cascade as minimally as possible. Our data indicate that in these conditions, using a Solacea™ dialyser allows safe reduction of the regular dose of systemic anticoagulant.
Other options in such a setting are local anticoagulation using citrate or calcium zero dialysate [12–15], or using heparin-coated membranes. Use of local anticoagulation with citrate is considered safe, but is logistically cumbersome in a chronic setting [12]. Use of heparin-coated membranes has been established in clinical practice, but the evidence to underpin this strategy is small [16, 17]. Of note, in our study, no dialysis sessions had to be terminated prematurely, but using a more sensitive gold standard technique to evaluate fibre blocking, we noted a substantial reduction in percentage of open fibres using FX800CORDIAX, whereas this was absent with Solacea™, even when applying a half dose of anticoagulation. In small, single-centre studies [18, 19] regional anticoagulation by either a heparin-coated membrane, regional citrate or regional citrate and calcium 0 has been evaluated, with varying success. Overall, the use of a Solacea™ membrane seems thus to be a promising, easily manageable, efficacious and safe strategy in conditions where no systemic anticoagulation can be used.
It is generally accepted that activation of the coagulation cascade is influenced by bio-incompatibility [20]. Our observation that, during a regular dialysis session, only a limited degree of fibre blocking occurred even with substantially reduced systemic anticoagulation seems to suggest good biocompatibility of the Solacea™ ATA™ membrane. Using citrate as local anticoagulant also inhibits clotting, but does not restore bio-incompatibility issues related to the membrane, and does not block the activation of white blood cells or complement [21, 22].
On a theoretical basis, differences in coagulation and fibre clotting could also be induced by differences in rheological properties of the blood because of large differences in protein content. However, within this population, no differences in baseline Alb concentrations were present, and there was also no indication of paraproteinemias, making this less likely. In addition, each patient was his own control, so the conclusions with regard to the behaviour of the different membranes would still hold.
Compared with events associated with actual clinical clotting of the extracorporeal circuit, less is known about the impact on dialyser performance and clearance of subclinical fibre blocking, i.e. without the need to terminate the dialysis session before the planned dialysis end. Using a reference technique to visualize individual fibres, this study indicates that fibre blocking can happen to a substantial degree without the need to stop the dialysis session, and even without being noticed. This study is, to our knowledge, the first to use the recently described micro-CT scan technique [6] to compare different dialyser types with regard to their capacity to induce clotting. It demonstrates that this sensitive technique can provide useful and clinically relevant information for the decision on which membrane type to select.
Patients with ESKD have a substantially increased cardiovascular risk as compared with age-matched persons without kidney disease. Cumulative retention of products in the middle molecular weight range 15–45 kDa substantially contributes to this enhanced cardiovascular mortality by inducing inflammation. Removal of possibly pro-inflammatory uraemic retention products is thus a potential strategy to reduce inflammation, and hopefully, to improve patient outcomes. It is, however, of importance to keep in mind that contact with the dialysis membrane itself can induce inflammation due to bio-incompatibility. Conventional high-flux dialysers do not efficiently remove molecules in the middle molecular weight range, such as β2M, tumour necrosis factor-α, interleukins, kappa and lambda FLCs, or myoglobin, resulting in their accumulation in patients with ESKD [1, 23–27]. HDF with post-dilution, however, does effectively result in enhanced removal of these middle molecules [28]. Our study indicates that the reduction ratios of kappa and lambda FLCs and of myoglobin were not different from Solacea™ as compared with FX800CORDIAX when used in post-dilution HDF with standard anticoagulation. However, the myoglobin extraction ratio was found to decrease, already at 60 min, in case of lower anticoagulation. The reduction ratios we have found for the FX800CORDIAX were compared with those reported previously by others using the same HDF parameters [25]. Using the Solacea™ in post-dilution HDF is most likely still inferior for removal of lambda and kappa FLCs than a medium cut off (MCO) membrane [25]. However, these MCO membranes result in a substantial Alb loss of >3.5 g per session with FX800CORDIAX. Alb loss with the Solacea™ membrane has been evaluated before and was reported to be between 1 and 3 g for a regular dialysis session using very sensitive analytical techniques [29, 30]. In our study, Alb loss in the dialysate was below the detection limit of our routine detection method for Alb in dialysate (0.9 mg/dL). As total dialysate volume spent during a regular dialysis session is around 200 L, this confirms that Alb loss should not be considered as clinically relevant problem when using these membranes in routine clinical practice. There was also no evidence for Alb adherence to the Solacea™ membrane in our experiments.
Whereas the small patient number (n = 10) can be considered as a limitation, this allowed us to use each patient as his own control over the four experimental regimens. Also, patients were dialysed only twice at the half dose of their regular anticoagulation regime.
As a strength, we have used a very sensitive reference method that allows us to measure coagulation in an objective way. Even using this sensitive method, little or no sign of fibre blocking was apparent when using the Solacea™ membrane.
In conclusion, the Solacea™ membrane seems to be ideal in conditions where systemic anticoagulation is prohibited as it outperforms polysulfone membranes under conditions of low systemic anticoagulation. Further research to explore whether this also reflects better biocompatibility, in general, are also warranted.
ACKNOWLEDGEMENTS
The authors are indebted to the dialysis nurses for their help during the clinical study, and to Sofie Vermeiren for her assistance in the fibre counting process.
FUNDING
Ghent University Hospital KOF 2015 grant for F.V., Ghent University funding BOF.AXP.2017.000007, and an unrestricted grant from Nipro Corporation (Osaka, Japan).
CONFLICT OF INTEREST STATEMENT
This study was financially supported with an unrestricted grant from Nipro Corporation, Osaka, Japan. F.V. received funding from Klinisch OnderzoeksFonds UZGent. W.V.B. and S.E. received travel grants from Baxter Healthcare and Fresenius Medical Care.
REFERENCES
- 1. Vanholder R, Pletinck A, Schepers E. et al. Biochemical and clinical impact of organic uremic retention solutes: a comprehensive update. Toxins 2018; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pletinck A, Glorieux G, Schepers E. et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 2013; 24: 1981–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Biesen W, Vanholder R, Schepers E. et al. The place of large pore membranes in the treatment portfolio of patients on hemodialysis. Contribut Nephrol 2017; 191: 168–177 [DOI] [PubMed] [Google Scholar]
- 4. Sunohara T, Masuda T.. Fundamental characteristics of the newly developed ATA membrane dialyzer. Contrib Nephrol 2017; 189: 215–221 [DOI] [PubMed] [Google Scholar]
- 5. Vanholder R, Ringoir S.. Bioincompatibility: an overview. Int J Artif Organs 1989; 12: 356–365 [PubMed] [Google Scholar]
- 6. Vanommeslaeghe F, Van Biesen W, Dierick M. et al. Micro-computed tomography for the quantification of blocked fibers in hemodialyzers. Sci Rep 2018; 8: 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bosch T, Schmidt B, Samtleben W, Gurland HJ.. Effect of protein adsorption on diffusive and convective transport through polysulfone membranes. Contribut Nephrol 1985; 46: 14–22 [DOI] [PubMed] [Google Scholar]
- 8. Rockel A, Hertel J, Fiegel P. et al. Permeability and secondary membrane formation of a high flux polysulfone hemofilter. Kidney Int 1986; 30: 429–432 [DOI] [PubMed] [Google Scholar]
- 9. Unger JK, Haltern C, Portz B. et al. Relation of haemofilter type to venous catheter resistance is crucial for filtration performance and haemocompatibility in CVVH–an in vitro study. Nephrol Dial Transplant 2006; 21: 2191–2201 [DOI] [PubMed] [Google Scholar]
- 10. Vanommeslaeghe F, De Somer F, Josipovic I. et al. Evaluation with micro-CT of different anticoagulation strategies during hemodialysis in patients with thrombocytopenia: a randomized crossover study. Artif Organs 2019; 43: 756. [DOI] [PubMed] [Google Scholar]
- 11.European Best Practice Guidelines Expert Group on Hemodialysis, European Renal Association. Section V. Chronic intermittent haemodialysis and prevention of clotting in the extracorporal system. Nephrol Dial Transplant 2002; 17 (Suppl 7): 63–71 [DOI] [PubMed] [Google Scholar]
- 12. Apsner R, Buchmayer H, Gruber D, Sunder-Plassmann G.. Citrate for long-term hemodialysis: prospective study of 1, 009 consecutive high-flux treatments in 59 patients. Am J Kidney Dis 2005; 45: 557–564 [DOI] [PubMed] [Google Scholar]
- 13. Gubensek J, Kovac J, Benedik M. et al. Long-term citrate anticoagulation in chronic hemodialysis patients. Ther Apher Dial 2011; 15: 278–282 [DOI] [PubMed] [Google Scholar]
- 14. Kreuzer M, Bonzel KE, Buscher R. et al. Regional citrate anticoagulation is safe in intermittent high-flux haemodialysis treatment of children and adolescents with an increased risk of bleeding. Nephrol Dial Transplant 2010; 25: 3337–3342 [DOI] [PubMed] [Google Scholar]
- 15. Schneider M, Thomas K, Liefeldt L. et al. Efficacy and safety of intermittent hemodialysis using citrate as anticoagulant: a prospective study. Clin Nephrol 2007; 68: 302–307 [DOI] [PubMed] [Google Scholar]
- 16. Islam MS, Hassan ZA, Chalmin F. et al. Vitamin e-coated and heparin-coated dialyzer membranes for heparin-free hemodialysis: a multicenter, randomized, crossover trial. Am J Kidney Dis 2016; 68: 752–762 [DOI] [PubMed] [Google Scholar]
- 17. Laville M, Dorval M, Fort Ros J. et al. Results of the HepZero study comparing heparin-grafted membrane and standard care show that heparin-grafted dialyzer is safe and easy to use for heparin-free dialysis. Kidney Int 2014; 86: 1260–1267 [DOI] [PubMed] [Google Scholar]
- 18. Evenepoel P, Dejagere T, Verhamme P. et al. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: a prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am J Kidney Dis 2007; 49: 642–649 [DOI] [PubMed] [Google Scholar]
- 19. Francois K, Wissing KM, Jacobs R. et al. Avoidance of systemic anticoagulation during intermittent haemodialysis with heparin-grafted polyacrilonitrile membrane and citrate-enriched dialysate: a retrospective cohort study. BMC Nephrol 2014; 15: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Sanctis LB, Stefoni S, Cianciolo G. et al. Effect of different dialysis membranes on platelet function. A tool for biocompatibility evaluation. Int J Artif Organs 1996; 19: 404–410 [PubMed] [Google Scholar]
- 21. Dhondt A, Vanholder R, Tielemans C. et al. Effect of regional citrate anticoagulation on leukopenia, complement activation, and expression of leukocyte surface molecules during hemodialysis with unmodified cellulose membranes. Nephron 2000; 85: 334–342 [DOI] [PubMed] [Google Scholar]
- 22. Dhondt A, Vanholder R, Waterloos MA. et al. Citrate anticoagulation does not correct cuprophane bioincompatibility as evaluated by the expression of leukocyte surface molecules. Nephrol Dial Transplant 1998; 13: 1752–1758 [DOI] [PubMed] [Google Scholar]
- 23. Vanholder R, De Smet R, Hsu C. et al. Uremic toxicity: the middle molecule hypothesis revisited. Semin Nephrol 1994; 14: 205–218 [PubMed] [Google Scholar]
- 24. Vanholder R, Van Laecke S, Glorieux G.. The middle-molecule hypothesis 30 years after: lost and rediscovered in the universe of uremic toxicity? J Nephrol 2008; 21: 146–160 [PubMed] [Google Scholar]
- 25. Kirsch AH, Lyko R, Nilsson LG. et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017; 32: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macias N, Vega A, Abad S. et al. Middle molecule elimination in expanded haemodialysis: only convective transport? Clin Kidney J 2019; 12: 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Prieto A, Vega A, Linares T. et al. Evaluation of the efficacy of a medium cut-off dialyser and comparison with other high-flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin Kidney J 2018; 11: 742–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meert N, Eloot S, Schepers E. et al. Comparison of removal capacity of two consecutive generations of high-flux dialysers during different treatment modalities. Nephrol Dial Transplant 2011; 26: 2624–2630 [DOI] [PubMed] [Google Scholar]
- 29. Maduell F, Rodas L, Broseta JJ. et al. Medium cut-off dialyzer versus eight hemodiafiltration dialyzers: comparison using a global removal score. Blood Purif 2019; 48: 167–174 [DOI] [PubMed] [Google Scholar]
- 30. Maduell F, Ojeda R, Arias-Guillen M. et al. A new generation of cellulose triacetate suitable for online haemodiafiltration. Nefrologia 2018; 38: 161–168 [DOI] [PubMed] [Google Scholar]