Abstract
Background
Antineutrophil cytoplasm antibody (ANCA)-associated vasculitis is a chronic relapsing and remitting autoimmune disease. Urinary soluble CD163 (usCD163) has been proposed as a biomarker of active renal vasculitis. We aimed to assess the potential usefulness of usCD163 for diagnosing renal relapse in patients with ANCA-associated glomerulonephritis.
Methods
One hundred and fifty-six samples from 47 patients with ANCA-associated glomerulonephritis belonging to two different cohorts (incident and prevalent) and 20 healthy controls were studied. Patients from the incident cohort were prospectively followed up, and usCD163 concentrations were measured every 3 months. Renal relapses were identified and changes in usCD163 concentrations were analysed.
Results
Normalized usCD163 concentrations were elevated at disease onset in all patients with active renal vasculitis, with a median concentration of 601 ng/mmol (interquartile range 221–1404 ng/mmol). On the other hand, usCD163 concentrations were undetectable among control patients with renal vasculitis in remission. Except for non-responders, usCD163 concentrations progressively decreased in all patients after treatment. In the presence of vasculitis relapse, there was a consistent increase in usCD163 concentrations, compared with previous values. The area under the receiver-operating characteristic curve of absolute and relative changes in usCD163 concentrations to identify relapse of ANCA-associated glomerulonephritis was 0.96 [95% confidence interval (CI) 0.91–1.00; P = 0.001] and 0.95 (95% CI 0.90–1.00; P = 0.001), respectively. Sensitivity and specificity for a relative increase of 20%, or an absolute increase of 20 ng/mmol, in usCD163 concentrations were 100% for both, and 89.3% and 87.5%, respectively. Urinary sCD163 concentrations significantly correlated with Birmingham Vasculitis Activity Score scores at Month 6 (r = 0.737; P = 0.006) and Month 12 (r = 0.804; P = 0.005).
Conclusions
usCD163 represents an accurate biomarker for the detection of active renal vasculitis and relapse. Its close association with disease activity provides additional information for monitoring treatment response.
Keywords: ANCA, biomarker, glomerulonephritis, vasculitis
INTRODUCTION
Antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) is characterized by small vessel inflammation and immune reactivity against the neutrophil and monocyte components proteinase 3 (PR3) and myeloperoxidase (MPO) [1–3]. Following immunosuppressive treatment, most patients with AAV undergo disease remission [4]. However, between 20% and 56% of patients have at least one relapse episode during evolution, usually within the first 5 years after disease onset [5–7], and relapses have been associated with increased morbidity and poorer survival [8].
Up to now, there have been no reliable activity markers available for vasculitis. ANCA titres do not closely follow disease activity, as patients in clinical remission frequently have persistent ANCA positivity, and serial measurements have been demonstrated to be limited in guiding individualized patient treatment [9]. Use of commonly measured inflammatory markers, such as C-reactive protein (CRP), is also limited due to a lack of specificity, as they are influenced by other diseases, including concomitant infection [10].
Soluble CD163 (sCD163), measured in the urine of patients with renal vasculitis, represents a very promising candidate biomarker. CD163 is a membrane protein localized on the surface of monocytes and macrophages [11]. The soluble form sCD163 is produced by enzymatic splitting of the ectodomain as a response to pro-inflammatory stimuli [12]. According to O’Reilly et al., activated macrophages infiltrate the glomeruli with incipient glomerulonephritis and secrete sCD163 into the urine [13]. In agreement with this hypothesis, the authors found a close association between increased urinary soluble CD163 (usCD163) concentrations and active renal vasculitis. The usCD163 concentrations in patients with acute vasculitis were significantly higher than in patients in the remission stage.
However, no prospective studies have been conducted to evaluate the usefulness and accuracy of this biomarker in patients suffering from vasculitis relapse, and information on the evolution of usCD163 concentrations during follow-up after immunosuppressant therapy is scarce. In this study, we evaluated the potential usefulness of usCD163 as a biomarker of renal activity and relapse in a prospective cohort of patients diagnosed with ANCA-associated glomerulonephritis. Additionally, we aimed to examine whether sCD163 measurements at onset and during follow-up could differentiate treatment response among these patients.
MATERIALS AND METHODS
Cohorts of AAV patients
One hundred and fifty-six samples from 47 patients with ANCA-associated glomerulonephritis belonging to two different cohorts (incident and prevalent) and 20 healthy controls were studied.
The incident cohort from four Spanish centres was composed of 24 adult patients who were diagnosed with AAV with renal involvement between 2016 and 2017 and prospectively followed up. Renal biopsy was performed at disease onset. Renal vasculitis was defined histologically by the presence of extracapillary proliferation associated with focal glomerular necrosis and/or small vessel vasculitis, in the absence of significant glomerular immune deposits [14]. Biopsies were reviewed by one nephropathologist (F.D.-C.) who was blinded to patient information, and renal samples were classified into histological subgroups according classification scheme of Berden et al.. Patients with secondary vasculitis were excluded. Clinical and sampling (for subsequent analysis) visits were scheduled at diagnosis, Month 1 and Month 2, and subsequently every 3 months. Urine samples were collected at each visit. Baseline samples were obtained before immunosuppressive treatment was commenced. Medical records and pathologic data were collected at the time of renal biopsy, including patient age, sex, dialysis requirement and ANCA serology. Microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and renal-limited vasculitis (RLV) were diagnosed according to the 2012 Chapel Hill Consensus Conference (CHCC) criteria [15]. Vasculitis disease activity was recorded using the Birmingham Vasculitis Activity Score (BVAS; Version 3) [16] at each patient visit. Disease manifestations were scored when they were attributable to active vasculitis and occurred in the previous month (new onset or worsening). Symptoms were considered as ‘persistent disease’ if they were due to active (but not new or worse) vasculitis and lasted <3 months. When disease manifestations were present for longer than 3 months, they were considered as ‘damage’, and therefore not BVAS-scored.
All patients were assessed every 3 months for haematuria (red blood cells per high-power field), urine protein/creatinine excretion ratio (g/g), serum creatinine (sCr) (mg/dL), estimated glomerular filtration rate (eGFR), as measured using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)-creatinine formula [17], and CRP concentration (mg/L). The immunosuppressive agents and response rates and relapses during follow-up were recorded. Complete remission was considered to be present when BVAS scores returned to zero, and absence of renal disease activity was indicated by stable or falling creatinine concentration and absence of red cell casts. Relapse was defined as the presence of active urine sediment and/or an increase in creatinine concentration by >30% attributable to active vasculitis.
The prevalent cohort was composed of 23 adult patients with ANCA-associated glomerulonephritis who were in remission and not receiving immunosuppressants or dialysis therapy; these patients were studied as controls. Additionally, 20 healthy controls with no renal or autoimmune diseases were also included. Healthy controls were recruited from volunteer patients who underwent ambulatory minor surgery and urine samples were collected before the surgical procedure was performed.
The study was approved by the Research Ethics Committees of the Fundación Hospital Alcorcon, and all recruits provided written informed consent.
usCD163 assays
Urine samples were collected by the patients in sterile cups and aliquoted without further manipulation, frozen at –80°C at each participating clinical site, shipped in dry ice to the central BioBank and stored at −80°C until subsequent use. usCD163 concentrations were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Human sCD163 DuoSet, DY1607, R&D Systems, city, country), according to the manufacturer’s instructions. In brief, urine samples were diluted 1:4 in the reagent diluent. usCD163 levels were measured in nanograms per millilitre (ng/mL) and normalized according to the urine creatinine concentration (mmol/L) that was previously determined using the Jaffe method [Creatinine (urinary) Colorimetric Assay Kit, 500701, Cayman Chemical, Ann Arbor, MI, US].
Statistical analysis
Qualitative data were expressed as absolute numbers and percentages. Quantitative data were expressed as the mean and standard deviation (SD) and, in the case of non-normal distribution, as the median and interquartile range (IQR). Comparisons were made with the t-test for normally distributed continuous variables, and the Mann–Whitney U test for non-normally distributed continuous variables. The Kolmogorov–Smirnov test was used to assess for normality. The study population was divided into tertiles, based on baseline usCD163 concentrations. Analysis of variance and chi-squared tests were used to compare variables among tertile groups. Correlations between usCD163 concentrations and BVAS scores were measured using the Spearman correlation coefficient in a time-stratified analysis.
In order to determine the usefulness of usCD163 concentrations to identify patients with renal relapse, relative changes in usCD163 concentrations, with respect to previous concentrations, were assessed, and the area under the receiver-operating characteristic (ROC) curve (AUC) was generated. The optimal cut-off values were calculated by maximizing the Youden index (sensitivity+specificity+1), validity index (sensitivity and specificity) and utility index (predictive positive value and predictive negative value). Mixed models were adjusted to examine longitudinal data. The models included time, treatment and their interaction as the fixed effect and time as a repeated measure. Bonferroni-adjusted significance tests were used for pair-wise comparisons. All hypothesis testing was two-tailed, and a P-value of <0.05 was considered statistically significant. All estimations were performed using the statistical analysis software STATA, version 14.1 (Stata Corp, College Station, TX, USA).
RESULTS
Patient demographic, serological and clinical characteristics in the incident cohort
Twenty-four patients were diagnosed with AAV renal flare during the study period and the longitudinal follow-up. Six patients (25%) had been previously diagnosed with ANCA-associated glomerulonephritis and the flares constituted a relapse. ANCA serology was positive in all patients; 79.2% showed activity against MPO, whereas activity against PR3 was identified in 20.8% of patients. Mean BVAS score at disease onset was 18.6 ± 3.3. Extra-renal involvement was present in 45.8% of participants and consisted of pulmonary involvement in 21% of cases, ORL involvement in 12.5% and cutaneous purpura, polyneuropathy and cardiac involvement (pericarditis) in 4.1%. According to the CHCC criteria, eight of the 24 patients (33.3%) were found to have MPA, three patients (12.5%) were identified as having GPA and 13 (54.1%) had RLV.
Participants were predominantly men (54.2%), and the median age was 70.4 ± 12.5 years. Median sCr at diagnosis was 3.4 mg/dL (IQR 2.5–3.8), and median eGFR 16.3 mL/min/1.73 m2 (IQR 8.3–32). Microhaematuria was present in all participants, and median proteinuria at diagnosis was 0.76 g/g (IQR 0.5–1.78). Of the 24 patients, 4 (16.7%) required acute dialysis at disease onset (Table 1). All patients received immunosuppressive treatment, with 62.5% receiving steroids plus cyclophosphamide, 29.1% receiving steroids plus rituximab and 8.3% (two cases) receiving steroids plus both cyclophosphamide and rituximab. Five patients (21%) were additionally treated with plasmapheresis and the median number of sessions received was 6 (IQR 4–6.5). Of the 24 patients, 21 (87.5%) were responsive to treatment. One patient who did not respond early to treatment died due to infection. All patients who achieved remission received maintenance therapy, with 66.6% receiving azathioprine and the remainder receiving rituximab. Median follow-up period was 15 months (9–22.5). Five patients (20.8%) experienced a renal relapse during follow-up. All relapses were treated with steroids plus rituximab.
Table 1.
Demographic and clinical characteristics of incident patients with ANCA-associated glomerulonephritis
| Variable | Total |
|---|---|
| n = 24 | |
| Mean age (years) (SD) | 70.4 (12.5) |
| Male, n (%) | 13 (54.2) |
| ANCA-positive, n (%) | 24 (100) |
| MPO, n (%) | 19 (79.2) |
| PR3, n (%) | 5 (20.8) |
| Relapser patients, n (%) | 6 (25) |
| Extra-renal involvement, n (%) | 11 (45.8) |
| Mean BVAS score (SD) | 18.6 (3.3) |
| Dialysis at onset, n (%) | 4 (16.7) |
| Inmunosuppressive therapy, n (%) | 24 (100) |
| Steroids + CYC, n (%) | 15 (62.5) |
| Steroids + RTX, n (%) | 7 (29.1) |
| Steroids + RTX + CYC, n (%) | 2 (8.3) |
| Treatment response, n (%) | 21 (87.5) |
| Relapses during follow-up, n (%) | 5 (20.8) |
| Median follow-up period (months) (IQR) | 15 (9–22.5) |
CYC, cyclophosphamide; RTX, rituximab.
With regard to histological characteristics, the mean percentage of glomeruli showing global sclerosis and the mean percentage of glomeruli with extracapillary proliferation were 20.2 ± 15% and 38.6 ± 29%, respectively. According to Berden’s histopathological classification, 33.3%, 27.7% and 38.8% of the biopsies were classified as mixed, crescentic and focal glomerulonephritis, respectively (Table 2).
Table 2.
Clinical and analytical characteristics of patients with ANCA-associated renal vasculitis according to baseline concentrations of normalized usCD163 (tertiles)
| Global | Tertile 1 | Tertile 2 | Tertile 3 | ||
|---|---|---|---|---|---|
| >321 ng/mmol | 321–1057 ng/mmol | >1057 ng/mmol | |||
| n = 24 | n = 8 | n = 8 | n = 8 | P-value | |
| Age (years) | 71 (62–83) | 70 (67–77) | 72 (60–74) | 66 (62.5–77.5) | 0.57 |
| ANCA, positive, n (%) | 24 (100) | 8 (33) | 8 (33) | 8 (33) | 0.32 |
| MPO, n (%) | 19 (79) | 7 (37) | 5 (26) | 7 (37) | |
| PR3, n (%) | 5 (21) | 1 (20) | 3 (60) | 1 (20) | |
| sCr (mg/dL) | 3.4 (2.5–3.8) | 1.8 (1.6–4.4) | 2.6 (1.7–4.7) | 4.3 (1.7–6.3) | 0.06 |
| eGFR (mL/min/1.73 m2) | 15.8 (7–29.6) | 32 (13–39) | 23 (13–34) | 15 (8.8–32.5) | 0.04 |
| Proteinuria UPCR (g/g) | 0.76 (0.5–1.78) | 0.6 (0.3–1.5) | 1.39 (0.85–1.7) | 2.2 (1.4–3) | 0.16 |
| Haematuria (RBCs/field) | 14 (4–44) | 6 (2.5–45) | 15 (6–25) | 25 (6–52) | 0.1 |
| Acute dialysis, n (%) | 4 (16.7) | 0 | 1 (13) | 3 (37) | 0.08 |
| Glomerulosclerosis (%) (IQR) | 18 (7.5–29) | 0 (0–17) | 18 (13.7–21.7) | 41 (34–45.7) | 0.01 |
| Treatment response, n (%) | 21 (87.5) | 8 (100) | 7 (87.5) | 6 (75) | 0.1 |
| Relapse, n (%) | 5 (20.8) | 2 (40) | 1 (20) | 2 (40) | 0.77 |
Data are presented as median (IQR) unless otherwise indicated.
Patient demographic and serological characteristics in the control cohort
Control vasculitis patients were predominantly men (59.6%), and the median age was 70.4 ± 11 years. ANCA serology was positive in 82.6% of patients; 15 patients (62.5%) showed activity against MPO, whereas activity against PR3 was identified in four patients (17.3%). According to the CHCC criteria, nine of these patients (39.1%) were classified as having MPA, three patients (13%) as having GPA and 11 patients (47.5%) as having RLV. All control patients were in complete remission (BVAS score of zero), and none received immunosuppression treatment. Median eGFR among control vasculitis patients was 36 mL/min (RIC, 32–49).
usCD163 concentrations among patients with active vasculitis
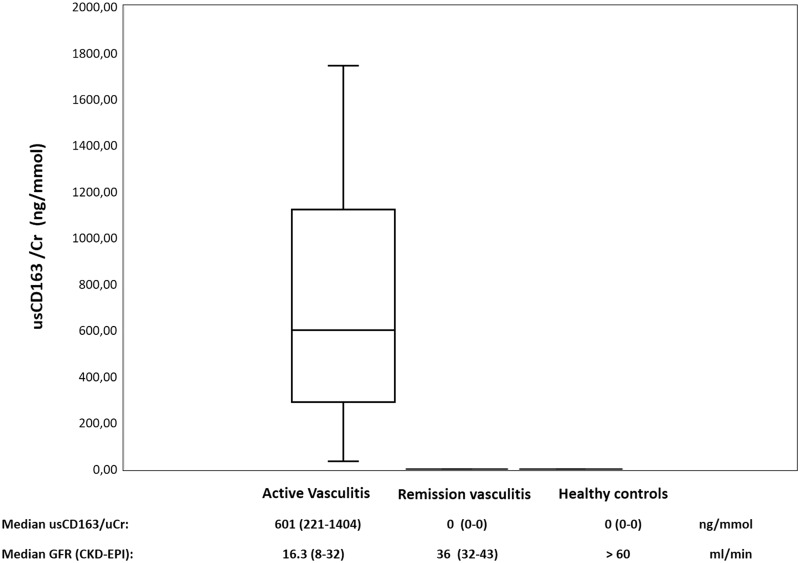
Normalized usCD163 concentrations, according to urine creatinine concentrations, were elevated at disease onset in all incident patients, and median concentration on admission was 601 ng/mmol (IQR 221–1404 ng/mmol). On the other hand, control patients with ANCA-associated glomerulonephritis who were in remission and healthy controls had undetectable concentrations of usCD163. Figure 1 shows usCD163 concentrations in the different study cohorts.
FIGURE 1.
Normalized usCD163 concentrations in patients with active renal vasculitis, those with renal vasculitis in remission and healthy controls.
Urinary sCD163 evaluation at diagnosis and clinical correlation
A wide range of usCD163 concentrations at diagnosis in patients with active renal vasculitis was observed. Thus, the study population was divided into three tertiles, according to baseline normalized usCD163 concentrations: Tertile 1: <321 ng/mmol; Tertile 2: 321–1057 ng/mmol; and Tertile 3: >1057 ng/mmol. Table 2 shows clinical and analytical characteristics of patients according to baseline usCD163 concentrations (tertiles). Patients in Tertile 3, with a higher concentration of usCD163 at diagnosis, had lower eGFR at disease onset (15 mL/min/1.73 m2, IQR 8.8–32) compared with patients in Tertile 1 (32 mL/min/1.73 m2, IQR 13–39) and Tertile 2 (23 mL/min/1.73 m2, IQR 13–34) (P = 0.04). Also, patients with a higher concentration of usCD163 (Tertile 3) showed a trend towards higher-grade haematuria and proteinuria at disease onset. With regard to histological lesions, a higher percentage of glomerulosclerosis in renal biopsies was observed among patients belonging to Tertile 3.
No differences were found in usCD163 concentrations among patients with de novo vasculitis (522 ng/mmol, IQR 186–1513) and those with a recurrence of AAV (660 ng/mmol, IQR 325–809). Also baseline concentrations of usCD163 at disease onset did not differ among patients according to ANCA serotype or histological subclass.
usCD163 as biomarker of activity and treatment response
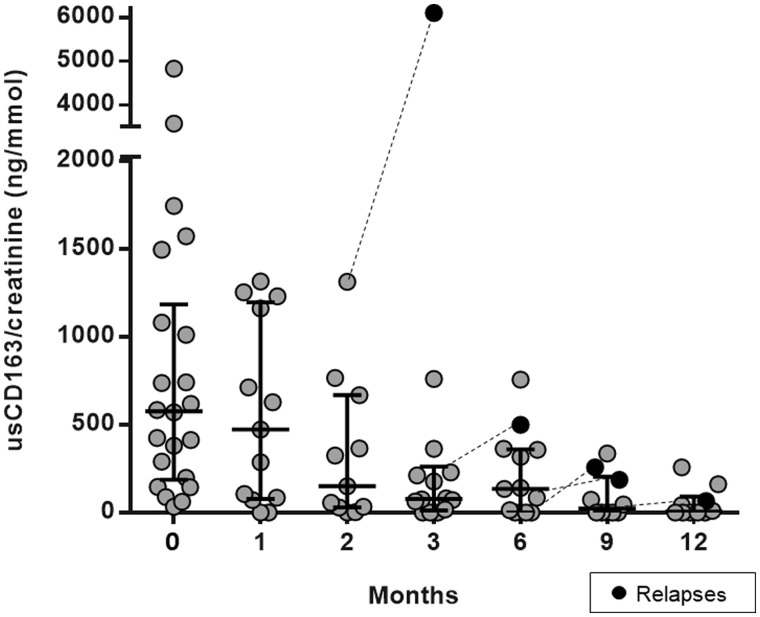
In the incident cohort, at Month 15 during follow-up, 16 patients were in remission, 2 patients did not respond to treatment and were on chronic dialysis, 1 patient died early due to infection and 5 patients experienced a renal relapse. Except for non-responder cases, all patients showed a progressive decrease in usCD163 concentrations during follow-up (Figure 2). All cases who did not respond to therapy belonged to Tertiles 2 and 3 with higher baseline concentrations of usCD163.
FGURE 2.
Changes in usCD163 concentrations following treatment and during follow-up of patients with ANCA-associated glomerulonephritis.
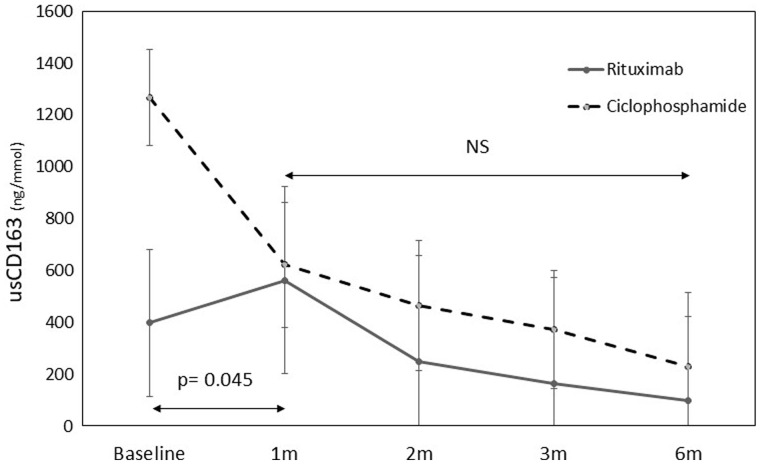
With regard to immunosuppression therapy, patients presenting with more severe renal injury often received treatment with cyclophosphamide. These patients had higher sCr concentrations at disease onset compared with those receiving rituximab (3.4 mg/dL, IQR 2.1–5.1 versus 1.9 mg/dL, IQR 1.4–2.8, respectively), and higher proteinuria (1.35 g/g, IQR 0.62–1.62 versus 0.8 g/g, IQR 0.5–2.7, respectively). These patients also presented with higher baseline concentrations of usCD163 compared with those treated with rituximab (1011 ng/mmol, IQR 289–1533 versus 412 ng/mmol, IQR 89–737, respectively; P = 0.09). As shown in Figure 3, cases treated with cyclophosphamide showed a more pronounced decrease in usCD163 concentrations in Month 1 compared with those treated with rituximab (P = 0.045). However, after Month 1, the decreasing slope of usCD163 concentrations was comparable between both groups. With regard to vasculitis activity, usCD163 concentrations showed a close correlation with BVAS scores at month 6 (r = 0.737; P = 0.006). No association was observed between usCD163 concentrations and changes in CRP concentrations and ANCA titres during follow-up.
FIGURE 3.
Evolution of median usCD163 concentrations according to the type of immunosuppressive therapy.
usCD163 as a biomarker of relapse
Five patients experienced a renal relapse during follow-up (Table 3). All patients who relapsed showed a significant increase in usCD163 concentrations compared with previous values. In one case (Patient 3), increased usCD163 concentrations were observed 3 months before the onset of haematuria and renal function impairment, thus predicting subsequent clinical relapse. Patients 1 and 5 did not show a significant rise in ANCA titres during relapse. Conversely, three patients had a significant increase in ANCA titres during follow-up without clinical significance. However, none of these patients showed an increase in usCD163 concentrations (data not shown). Additionally, in all four relapsing patients with a previous diagnosis of ANCA-associated glomerulonephritis before enrolment, usCD163 was undetectable using BioBank samples available within 6 months prior to renal flares. Only two patients showed an increase in usCD163 concentrations during follow-up without experiencing a vasculitis relapse. However, these increases were mild and transient in both cases.
Table 3.
Serological and biochemical changes in patients with a relapse of ANCA-associated glomerulonephritis
| Baseline | Month 1 | Month 3 | Month 6 | Month 9 | Month 12 | |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| sCr (mg/dL) | 1.7 | 1.5 | 1.3 | 1.2 | 1.4* | 1.5 |
| Haematuria (RBC/HPF) | 60 | 0 | 0 | 0 | 30 | 0 |
| ANCA titres (U/L) | 80.0 | 29.0 | 26.0 | 46.0 | 49.0 | 18.0 |
| usCD163/Cr (ng/mmol) | 199.4 | 0.0 | 0.0 | 0.0 | 258.4 | 0.0 |
| Patient 2 | ||||||
| sCr (mg/dL) | 1.9 | 1.3 | 1.4 | 0.9 | 1.3* | 2.8 |
| Haematuria (RBCs/HPF) | 30 | 4 | 4 | 0 | 4 | 30 |
| ANCA titres (U/L) | 130.0 | 63.0 | 1.4 | 0.6 | 4.0 | 134.0 |
| usCD163/Cr (ng/mmol) | 3580.6 | 1231.2 | 177.2 | 84.8 | 186.7 | 1490.6 |
| Patient 3 | ||||||
| sCr (mg/dL) | 2.6 | 3.0 | 2.4 | 2.0 | 1.7 | 2.1* |
| Haematuria (RBCs/HPF) | 10 | 6 | 0 | 0 | 0 | 4 |
| ANCA titres (U/L) | 32.0 | 32.0 | 0.0 | 1.0 | 2.5 | 11.0 |
| usCD163/Cr (ng/mmol) | 569.8 | 150.6 | 71.4 | 14.8 | 46.5 | 67.0 |
| Patient 4 | ||||||
| sCr (mg/dL) | 4.4 | 1.7 | 1.3 | 1.5* | 1.3 | 1.1 |
| Haematuria (RBCs/HPF) | 20 | 8 | 8 | 20 | 4 | 4 |
| ANCA titres (U/L) | 134.0 | 18.0 | 6.2 | 134.0 | 134.0 | 66.0 |
| usCD163/Cr (ng/mmol) | 425.5 | – | 212.3 | 500.0 | 336.2 | 257.4 |
| Patient 5 | ||||||
| sCr (mg/dL) | 6.4 | 4 | 6.2* | 4 | 3.4 | – |
| Haematuria (RBCs/HPF) | 60 | 30 | 60 | 30 | 30 | – |
| ANCA titres (U/L) | 113.0 | 15.0 | 6.4 | 3.5 | 1.8 | – |
| usCD163/Cr (ng/mmol) | 1572.5 | 1313.8 | 6104.2 | 756.1 | – | – |
RBCs/HPF, red blood cells per high-power field. * Relapse.
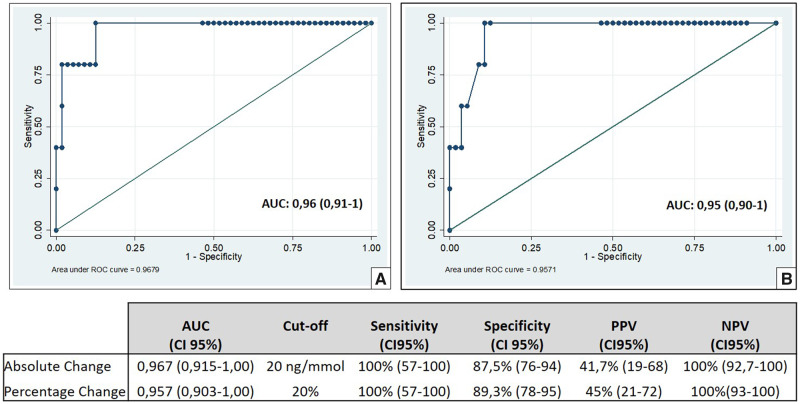
In order to assess whether usCD163 could identify the presence of relapse, we estimated the AUC using absolute [AUC 0.96; confidence interval (CI) 95% 0.91–1.00; P = 0.001] and relative (AUC 0.95, CI 95% 0.90–1.00; P = 0.001) intraindividual changes in usCD163 concentrations, with respect to previous values (Figure 4). The ROC curve-derived optimal cut-off values were 20 μg/mmol for usCD163 absolute changes and 20% for usCD163 relative changes. Use of these cut-off values resulted in 100% sensitivity for both, and a specificity of 87.5% and 89.3%, respectively. These results demonstrated that usCD163 measurements at each patient visit could robustly detect the occurrence of relapse.
FIGURE 4.
ROC curve analyses of absolute (A) and relative (B) changes in usCD164 concentrations, with respect to previous concentrations, to identify renal vasculitis relapse.
DISCUSSION
AAV is a chronic relapsing and remitting autoimmune disease. It is difficult to accurately identify renal relapse since changes in ANCA titres do not provide reliable information and renal impairment in these patients can be due to other clinical conditions such as infection or drug toxicity [6]. Therefore, relapses are often diagnosed too late, when severe glomerular damage is already established. Conversely, false relapse can induce overtreatment and the occurrence of possible unnecessary secondary events.
Recently, use of usCD163 measurements was assessed for diagnosing active vasculitis in patients with renal involvement, compared with AAV patients in remission or active AAV patients with extra-renal involvement [18]. In all these clinical scenarios, diagnosis of active ANCA-associated glomerulonephritis was possible with the use of normalized usCD163 concentrations. O’Reilly et al. [13] established an optimum cut-off value of 300 ng/mmol to accurately detect active renal vasculitis with a sensitivity of 83% and a specificity of 96%. Additionally, use of usCD163 measurements was also evaluated in the identification of active renal vasculitis compared with healthy patients, those with acute kidney injury or sepsis in the intensive care unit (ICU) and those with other glomerulonephritides. Although they found that usCD163 measurements had good sensitivity and specificity for diagnosing ANCA-associated glomerulonephritis, increased concentrations of usCD163 were also observed in 23% of patients with other glomerulonephritides. Therefore, these results somewhat restricted the use of usCD163 measurements for diagnosing ANCA-associated glomerulonephritis compared with other glomerular injuries. On the other hand, Moran et al. [18] noted a sensitivity of 79.5% and a specificity of 67.3%, although their cut-off value was 72.9 ng/mmol. However, these studies analysed heterogenous and different cohorts for each clinical condition, which could explain the different cut-off values observed between the studies.
In our study, we also demonstrated that usCD163 can accurately distinguish between patients with active renal vasculitis and those in remission. Also, in this study, high concentrations of usCD16 indicating retention as a result of renal impairment were excluded, since the majority of control patients with vasculitis who were in remission had some degree of renal insufficiency and showed undetectable concentrations of usCD163. This is the first prospective and longitudinal cohort study assessing the use of usCD163 to diagnose renal relapses in ANCA-associated glomerulonephritis. In our cohort, a wide range of baseline usCD163 concentrations, as well as great variability in intra-individual changes in usCD163 concentrations during follow-up, were observed. Therefore, we were not able to identify an absolute reference value for usCD163 that could identify the presence of renal relapse. However, a dynamic approach considering absolute and relative intra-individual changes in usCD163 concentrations allowed us to assess cut-off values for use in clinical practice. We found that an absolute increase of 20 μg/mmol or a relative increase of 20% in usCD163 concentrations, compared with previous values, identified renal relapse with excellent sensitivity and specificity, thus confirming accurate detection of renal relapses by using usCD163 measurements. Of note, one patient in our study showed an increase in usCD163 concentrations 3 months before clinical and biochemical evidence of renal relapse, suggesting that the rise in usCD163 concentrations predicted the onset of haematuria and worsening renal function. This makes for an interesting finding, since early identification of active disease using usCD163 measurements could allow treatment planning before more severe damage is established. Interestingly, two patients in the study had a renal relapse without an increase in ANCA titres, but with a significant increase in usCD163 concentrations. Conversely, three patients had a significant increase in ANCA titres during follow-up without relapsing, none of whom showed a rise in usCD163 concentrations. Therefore, our study also demonstrates that usCD163 measurements could be used to distinguish between the presence and absence of renal relapse with increasing ANCA titres.
Further, usCD163 concentrations can also provide useful information about disease severity and treatment response. In our cohort, higher baseline concentrations of usCD163 at disease onset were associated with lower eGFR and greater requirement for acute dialysis, thus identifying those patients with more severe injury who could benefit from a more aggressive induction therapy. With regard to treatment response, usCD163 concentrations progressively decreased following therapy in all patients achieving remission, and conversely, usCD163 concentrations did not change in the first 3 months in non-responder patients. Therefore, usCD163 measurements might guide treatment decisions, since a change in treatment approach could be considered in cases with persistently high usCD163 concentrations after 3 months of therapy. A different pattern of reduction in usCD163 concentrations in the first month was observed in patients treated with cyclophosphamide and rituximab. This could be due to different effects of both immunomodulators on renal macrophage infiltration [19–21] and could explain why induction regimens based on a combination of rituximab with low doses of cyclophosphamide have shown excellent results in recent studies [22, 23]. Finally, in our study, we observed a good correlation between BVAS scores and usCD163 concentrations during follow-up, suggesting that usCD163 measurements could be useful for monitoring treatment response, independently of the immunosuppression regimen used.
A limitation of our study is the inclusion of a small study population, which meant that only a limited number of relapses were evaluated. Therefore, further studies including larger series of patients with renal relapse should be desirable to confirm our findings. However, the prospective and longitudinal design of our study allowed us to accurately determine usCD163 concentrations during follow-up of patients who had a renal vasculitis relapse. This study also helps to validate the use of usCD1163 as a biomarker of disease activity in a population mainly composed of MPO-ANCA patients.
In conclusion, usCD163 is an accurate and useful biomarker to detect renal relapses in patients with AAV, in addition to its use in the diagnosis of AAV with renal involvement. Measurement of usCD163 concentrations also provides relevant information about treatment response and could help guide immunosuppression therapy in ANCA-associated glomerulonephritis.
ACKNOWLEDGEMENTS
The authors thank the Hospital Universitario Fundación Alcorcón BioBank staff for their assistance with sample collection and processing, and Elia Pérez from the Statistical Unit of Hospital Fundación Alcorcon for her assistance with data analysis.
FUNDING
This study was funded by a Research Grant from Hospital Fundacion Alcorcon, Fundación Renal Iñigo Alvárez de Toledo, Sociedad Madrileña de Nefrología and REDinREN (RD016/0021) Instituto de Salud Carlos III.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Falk RJ, Terrell RS, Charles LA. et al. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 1990; 87: 4115–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao H, Heeringa P, Hu P. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002; 110: 955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Little MA, Smyth CL, Yadav R. et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood 2005; 106: 2050–2058 [DOI] [PubMed] [Google Scholar]
- 4. Pagnoux C, Hogan SL, Chin H. et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Comparison of two independent cohorts. Arthritis Rheum 2008; 58: 2908–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flossmann O, Berden A, de Groot K. et al. ; European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011; 70: 488–494 [DOI] [PubMed] [Google Scholar]
- 6. Lionaki S, Blyth ER, Hogan SL. et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum 2012; 64: 3452–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weidner S, Geuss S, Hafezi-Rachti S. et al. ANCA-associated vasculitis with renal involvement: an outcome analysis. Nephrol Dial Transplant 2004; 19: 1403–1411 [DOI] [PubMed] [Google Scholar]
- 8. de Joode AAE, Sanders JSF, Stegeman CA.. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol 2013; 8: 1709–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomasson G, Grayson PC, Mahr AD. et al. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford) 2012; 51: 100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomasson G, Davis JC, Hoffman GS. et al. Brief report: the value of a patient global assessment of disease activity in granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum (Munch) 2014; 66: 428–432 [DOI] [PubMed] [Google Scholar]
- 11. Fabriek BO, Dijkstra CD, van den Berg TK.. The macrophage scavenger receptor CD163. Immunobiology 2010; 210: 153–160 [DOI] [PubMed] [Google Scholar]
- 12. Zhao L, David MZ, Hyjek E. et al. M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol 2015; 10: 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Reilly VP, Wong L, Kennedy C. et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2016; 27: 2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris AA, Falk RJ, Jennette JC.. Crescentic glomerulonephritis with a paucity of glomerular immunoglobulin localization. Am J Kidney Dis 1998; 32: 179–184 [DOI] [PubMed] [Google Scholar]
- 15. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65: 1–11 [DOI] [PubMed] [Google Scholar]
- 16. Mukhtyar C, Lee R, Brown D. et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68: 1827–1832. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moran SM, Monach PA, Zgaga L. et al. Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2020; 31: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolawole AO, McCorkle FM.. Cyclophosphamide effects on avian macrophages in vitro. J Immunol 2007; 178: 101 [Google Scholar]
- 20. Santosuosso M, Divangahi M, Zganiacz A. et al. Reduced tissue macrophage population in the lung by anticancer agent cyclophosphamide: restoration by local granulocyte macrophage-colony-stimulating factor gene transfer. Blood 2002; 99: 1246–1252 [DOI] [PubMed] [Google Scholar]
- 21. Toubi E, Kessel A, Slobodin G. et al. Changes in macrophage function after rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2007; 66: 818–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McAdoo SP, Medjeral-Thomas N, Gopaluni S. et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant 2019; 34: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pepper RJ, McAdoo SP, Moran SM. et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 2019; 58: 260–268 [DOI] [PubMed] [Google Scholar]